Jul. 11, 2008 Research Highlight Biology
Transport proteins make special deliveries
Two proteins responsible for ushering molecular cargo into the nucleus also play an unexpected role in making targeted deliveries during cell division
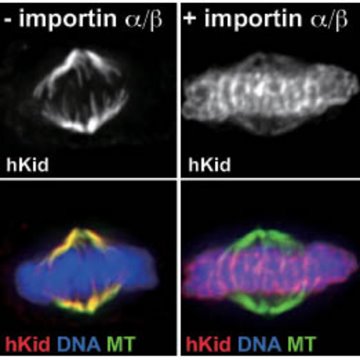 Figure 1: Disruption of hKid association with mitotic chromosomes in the absence of importin-α and -β. In the absence of the importins, hKid remains preferentially bound to the mitotic spindles (top left); following the reintroduction of importin-α and –β, hKid dissociates from the spindles and binds to the chromosomes, which are aligned at the center of the dividing cell (top right). The bottom panels show the same two images with distinct fluorescent labels for hKid (red), chromosomal DNA (blue), and the spindle microtubules (green). Reproduced from Ref. 1 © 2008 The Rockfeller University Press
Figure 1: Disruption of hKid association with mitotic chromosomes in the absence of importin-α and -β. In the absence of the importins, hKid remains preferentially bound to the mitotic spindles (top left); following the reintroduction of importin-α and –β, hKid dissociates from the spindles and binds to the chromosomes, which are aligned at the center of the dividing cell (top right). The bottom panels show the same two images with distinct fluorescent labels for hKid (red), chromosomal DNA (blue), and the spindle microtubules (green). Reproduced from Ref. 1 © 2008 The Rockfeller University Press
Any cytoplasmic protein requiring access to the nucleus typically needs an escort, in the form of specialized proteins that bind to cargo molecules and mediate their entry through pores in the nuclear envelope.
Two proteins involved in this trafficking process are the importins, α and β, which form a complex with target proteins and then shuttle them into the nucleus. Once inside, the importin–cargo complex is bound by the protein-nucleotide complex Ran-GTP, which triggers cargo release and the subsequent shuttling of the importins back into the cytoplasm.
During the early stages of mitosis, the process of cell division, the nuclear envelope disintegrates and the chromosomes are aligned in the center of the dividing cell by assemblies of long protein filaments known as spindles. One of the proteins that manage this process is hKid, which binds both to the spindles and to the chromosomes themselves.
Previous research has shown that hKid is transported by importins, but it has remained unclear how this association is relevant to the mitotic process. “Nobody had thought about importins having any role in targeting proteins to mitotic chromosomes, especially when there is no nuclear envelope present,” explains Naoko Imamoto, an investigator at the RIKEN Advanced Science Institute (formerly the Discovery Research Institute) in Wako. Nonetheless, this surprising finding is exactly what Imamoto’s team uncovered in their most recent work1.
They identified two nuclear localization signals (NLS)—the ‘address labels’ used by importins—on hKid. Without these NLS, or in the absence of the importin proteins, the ability of hKid to stably and efficiently associate with chromosomes was markedly reduced in mitotic cells (Fig. 1). Under these conditions, hKid instead showed an increased preference for binding the spindles, suggesting that the importins serve a double role: disrupting hKid spindle-binding, and targeting the protein to the chromosomes. They subsequently showed that this process is in turn expedited by the local production of Ran-GTP at the chromosome, which enables efficient release of hKid once the importin complex is appropriately positioned nearby.
Imamoto suggests that these findings may be part of a bigger picture, in which transport proteins are not merely ushers for mitosis-related factors, but deliverymen as well. “This is a new phenomenon,” she says. “We certainly think that the system found in this current study—the targeting of proteins onto mitotic chromosomes by transport factors—could also be adopted by many other mitotic chromosomal proteins. Therefore, we will examine the generality of this phenomenon.”
References
- 1. Tahara, K., Takagi, M., Ohsugi, M., Sone, T., Nishiumi, F., Maeshima, K., Horiuchi, Y., Tokai-Nishizumi, N., Imamoto, F., Yamamoto, T., Kose, S. & Imamoto, N. Importin-β and the small guanosine triphosphatase Ran mediate chromosome loading of the human chromokinesin Kid. Journal of Cell Biology 180, 493–506 (2008). doi: 10.1083/jcb.200708003
