Oct. 24, 2008 Research Highlight Biology
Cutting loose
New research clarifies how cells rearrange from two-dimensional sheets into three-dimensional structures during embryonic development
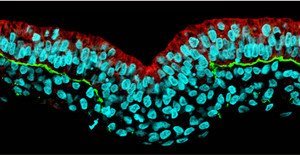 Figure 1: Cell nuclei have been stained cyan, with red and green stain indicating the presence of RhoA and laminin, respectively, illustrating the loss of expression RhoA that coincides with the loss of the basement membrane. Reprinted from Ref. 1 © 2008 Macmillan Publishers Ltd
Figure 1: Cell nuclei have been stained cyan, with red and green stain indicating the presence of RhoA and laminin, respectively, illustrating the loss of expression RhoA that coincides with the loss of the basement membrane. Reprinted from Ref. 1 © 2008 Macmillan Publishers Ltd
Early in development, vertebrate embryos transition from being simple balls of cells to more complex structures comprising three distinct cellular layers, each of which will give rise to a distinct subset of tissues. Part of this transitional process, called gastrulation, involves the carefully choreographed migration of the cells that form the mesodermal layer—the precursor to bones and muscles, among other tissues.
An important step in mesoderm formation is the epithelial–mesenchymal transition (EMT), in which two-dimensional (epithelial) sheets of cells rearrange into more complex three-dimensional (mesenchymal) structures. EMT is itself a complex multi-stage process, and even after decades of research, ambiguity remains about how it is coordinated.
Now, new findings from a team at the RIKEN Center for Developmental Biology in Kobe, led by Guojun Sheng and postdoctoral fellow Yukiko Nakaya, has provided valuable insights into the initial stages of EMT1. Earlier research by Nakaya had shown interesting effects of changes in expression of a protein called RhoA on mesoderm formation, and so the team decided to follow up by examining its role in EMT in developing chick embryos.
Sheng, Nakaya and colleagues found that EMT appears to begin with the breakdown of the basement membrane (BM), a matrix of proteins including laminin and fibronectin that provide structural support for epithelial sheets. They also found that retention of the BM within the epiblast—the embryonic cells actively involved in gastrulation—coincides with expression of RhoA at BM-facing cell surfaces; conversely, the absence of such RhoA expression in mesoderm precursor cells provides an indicator of BM disintegration (Fig. 1).
These findings were further supported by experiments in which RhoA expression was boosted or reduced, revealing a direct dependence of BM stabilization on local RhoA expression. This appears to result in part from RhoA’s effects on microtubules, long protein-based cables that compose part of the cellular infrastructure. The researchers found that loss of RhoA activity specifically disrupts microtubules near the BM-facing surface of epiblast cells, effectively severing their anchors to the BM as a logical first step before their reformation into more sophisticated three-dimensional assemblies.
Sheng was pleased to observe that their observed mechanism fits into a rational model for embryonic development. “The most surprising outcome,” he says, “is that the sequential steps of EMT in gastrulation fit beautifully with how one would imagine the mesoderm cells should come off from the epiblast.”
References
- 1. Nakaya, Y., Sukowati, E.W., Wu, Y. & Sheng, G. RhoA and microtubule dynamics control cell-basement membrane interaction in EMT during gastrulation. Nature Cell Biology 10, 765–775 (2008). doi: 10.1038/ncb1739
