Nov. 28, 2008 Research Highlight Biology
Rebuilding the brain
A method for deriving complex neuronal tissues from embryonic stem cells could yield major benefits for clinical research and the development of new therapeutics
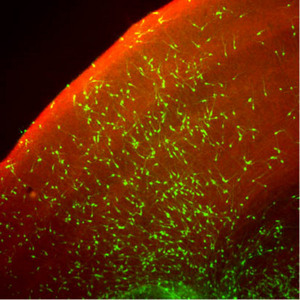 Figure 1: When cocultured with mouse brain slices, neuronal precursors derived from SFEBq cultures (green) develop into neurons that integrate with existing cerebral cortical structures. Reproduced, with permission, from Ref. 1 © 2008 Elsevier
Figure 1: When cocultured with mouse brain slices, neuronal precursors derived from SFEBq cultures (green) develop into neurons that integrate with existing cerebral cortical structures. Reproduced, with permission, from Ref. 1 © 2008 Elsevier
The great hope for embryonic stem cell (ESC) research is that scientists will be able to develop effective strategies for coaxing these cells to develop into a wide variety of mature cell types and tissues, which could in turn be used for clinical studies or even outright transplantation into patients.
As one might expect, the brain poses a particular challenge, being composed of a diverse array of cell types arranged in highly complex and specialized structures. There have been some promising signs of early progress, however. For instance, Yoshiki Sasai’s team at the RIKEN Center for Developmental Biology in Kobe recently described a technique for stem cell cultivation, SFEB, which enables the reliable derivation of a variety of cerebral precursor cell types from cultured ESCs1.
At the same time, SFEB has shown only limited efficiency in obtaining some particular cell types, such as cerebral cortical neurons—the cells that mediate many of the mammalian brain’s highest-order functions, including conscious thought and sensory processing.
To improve their method, Sasai and colleagues introduced a new twist—cultivating dissociated mouse ESCs under conditions that favor unrestricted aggregation in three dimensions, rather than in adherent cultures on a flat surface2. The results of their ‘quick aggregation’ SFEB (SFEBq) method are striking—nearly 95% of the resulting neuronal progenitors differentiated into sheets of neuroepithelial cells, brain tissue precursors with a distinctive polarized structure, in which the ‘upper’ and ‘lower’ segments of the cells exhibit distinctive physical characteristics.
Getting with the program
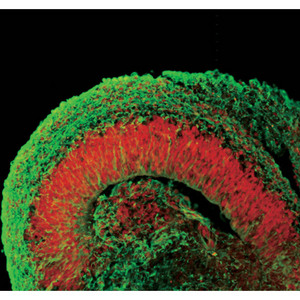 Figure 2: Human ESCs cultured via the SFEBq method develop into structures that resemble the early fetal cortex. Cells have been fluorescently stained for a cerebral marker (red) and to indicate cortical neurons (green).
Figure 2: Human ESCs cultured via the SFEBq method develop into structures that resemble the early fetal cortex. Cells have been fluorescently stained for a cerebral marker (red) and to indicate cortical neurons (green).
With further cultivation, these sheets reformed into spherical clusters that subsequently yielded functional cortical neurons, both in culture and after transplantation into mouse brains (Fig. 1). By further tweaking the culture conditions to include various cell signaling factors, Sasai’s team was even able to derive neuronal subtypes from specific cortical structures, such as the olfactory bulb, as well as noncortical cells.
These cultures also behaved similar to embryonic cortical tissue, displaying spontaneous signaling activity and even network behavior. “The induced cortical tissues started to exhibit synchronized neural activity over a range of more than one millimeter,” he says, “which is characteristic of the neural activity seen in the neonatal cortex.”
But there were other surprises as well. The cerebral cortex is composed of six parallel layers, each comprising a different cellular ecosystem of distinctive neuronal subtypes; these layers develop in a specific order, with outermost layer I appearing first, after which layers VI through II develop sequentially in an ‘inside-out’ manner. By examining changes over time in gene expression profiles in their SFEBq cultures, the researchers found that their ESC-derived cortical neuron progenitors were naturally entering into a stepwise developmental program of layer formation that closely mirrors normal embryonic cortical development (Fig. 2). “This is self-formation of a highly ordered pattern from patternless cells, which is quite impressive to me,” says Sasai. “In other words, it is the programmed nature of these cells to form such structures.”
The SFEBq cultures recapitulated the development of four of the six cortical layers in terms of timing, and the researchers were able to modify their culture protocol to isolate layer-specific neuron types. However, these layers did not exhibit appropriate physical arrangement, and two of the layers never developed at all, suggesting that considerably more remains to be understood about how this process is regulated. “It was really wonderful to see successful formation of the four-layered tissue,” says Sasai. “However, for basic scientists like me, it is even more exciting to understand why the next steps of corticogenesis fail to occur. What is missing? It may take a really long time to address this question.”
Sasai believes this work will provide a valuable starting point for exploring a number of mysteries about the early stages of cerebral development, and his team is actively working to develop new imaging tools that will allow them to monitor these SFEBq-derived cell clusters in real-time, and to attempt to better understand the processes involved in cortical differentiation.
A new approach to stem cell research
More generally, however, the ability to recapitulate and even control the complex developmental processes underlying tissue formation in culture is exciting from both a research and a medical perspective, and the successful extension of this and other techniques for neuronal cultivation and manipulation may ultimately be a game-changer for the field of neuroscience. “So far, stem cell projects have been aiming at the generation of useful cells,” says Sasai. “The present work demonstrates a new stage: the generation and use of functional tissues. This makes a large difference from working with ‘just cells’ in applications relating to drug discovery, toxicology, disease pathogenesis, and—in the long-term—tissue replacement, because such tissues mimic the in vivosituation much better than simple cell cultures.”
Of course, many of the more ambitious objectives relating to in vitroengineering of functional, transplantable nervous tissue currently remain solidly in the realm of science fiction. Nevertheless, Sasai perceives complicated ethical questions that may lie ahead as these technologies continue to advance, especially regarding how far scientists should be willing to pursue projects related to engineering of human brain tissue. “So far,” he says, “it is not difficult to answer, because the tissue we can produce right now is still very immature—far from our own cortical tissues and their capacity for higher brain functions. However, in the long-term future, a day may come when we really need to consider the question more seriously.”
References
- 1. Watanabe, K., Kamiya, D., Nishiyama, A., Katayama, T., Nozaki, S., Kawasaki, H., Watanabe, Y., Mizuseki, K. & Sasai, Y. Directed differentiation of telencephalic precursors from embryonic stem cells. Nature Neuroscience 8, 288–296 (2005). doi: 10.1038/nn1402
- 2. Eiraku, M., Watanabe, K., Matsuo-Takasaki, M., Kawada, M., Yonemura, S., Matsumura, M., Wataya, T., Nishiyama, A., Muguruma, K. & Sasai, Y. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3, 519–532 (2008). doi: 10.1016/j.stem.2008.09.002
About the Researcher
Yoshiki Sasai

Yoshiki Sasai was born in Hyogo, Japan in 1962 and received his MD from Kyoto University School of Medicine in 1986. From 1986 to 1988, he performed an internship in general and emergency medicine. In 1993, he earned a PhD from the same university for work on neural specific transcriptional regulators. From 1993 to 1996, he was a visiting research fellow at UCLA School of Medicine, and then appointed as an associated professor at Kyoto University School of Medicine. From 1998 to 2003, he served as a professor at Institute for Frontier Medical Sciences, Kyoto University, while becoming a group director at the RIKEN Center for Developmental Biology in 2000. He has been concurrently serving as an affiliated professor at Kyoto University School of Medicine since 2003.
