Jun. 4, 2010 Research Highlight Biology
Making the right contacts to get ahead
A set of mutant yeast strains allows researchers to identify structural elements that help motor proteins to get moving
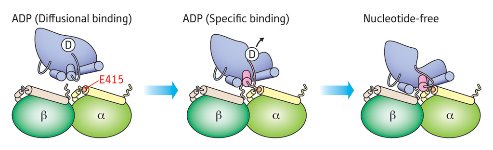 Figure 1: Schematic of the two-step association of kinesin (blue) with microtubules as revealed by mutational analysis. The initial interaction of kinesin-ADP with residue E415 of α-tubulin (left) triggers a conformational change that leads to release of ADP (middle), and the nucleotide-free kinesin subsequently participates in additional stabilizing interactions with residues on the β-tubulin subunit (right). © 2010 Etsuko Muto
Figure 1: Schematic of the two-step association of kinesin (blue) with microtubules as revealed by mutational analysis. The initial interaction of kinesin-ADP with residue E415 of α-tubulin (left) triggers a conformational change that leads to release of ADP (middle), and the nucleotide-free kinesin subsequently participates in additional stabilizing interactions with residues on the β-tubulin subunit (right). © 2010 Etsuko Muto
Cells are crisscrossed by microtubules, protein cables that provide essential infrastructure and serve as ‘highways’ for moving molecular cargoes. Motor proteins, such as kinesin that travels along microtubules via a multi-step ‘walking’ mechanism, effectively drive this transport. The broad strokes of this process are well understood generally, but new work from Etsuko Muto and Seiichi Uchimura of the RIKEN Brain Science Institute in Wako in collaboration with physicists at Waseda University, Tokyo, has revealed valuable new details about how microtubule interactions facilitate kinesin movement1.
Kinesin is associated with the nucleotide molecule adenosine diphosphate (ADP) when it first binds microtubules, after which it undergoes a structural change that triggers release of ADP and enables interaction with adenosine triphosphate (ATP). Subsequent enzymatic processing of ATP into ADP triggers additional structural changes, causing kinesin to move forward along the microtubule while also returning the protein to its initial ADP-bound state.
Microtubules are composed of dimers of the protein α- and β-tubulin, but eukaryotic cells can have numerous different tubulin subtypes, making it challenging to investigate molecular-level details of kinesin–tubulin interaction. To overcome this problem, Muto and Uchimura developed yeast strains that express only a single subtype each of α- and β-tubulin, thus enabling simple screening of the effects of individual tubulin mutations. In their most recent work, they have used this approach to extensively characterize points of interaction between kinesin and microtubules by generating 36 yeast strains with individual mutations in either tubulin subunit.
Their data suggest that α-tubulin is primarily responsible in the initial association with kinesin-ADP, with β-tubulin providing important stabilizing interactions following the release of ADP (Fig. 1). The researchers were particularly surprised to note that mutations targeting one highly conserved glutamate (E415) in α-tubulin caused a five-fold reduction in kinesin enzymatic activity, apparently by impairing binding-induced release of ADP. “Our results indicate that kinesin binding to residue E415 in α-tubulin transmits a signal to the kinesin nucleotide pocket, triggering its conformational change and leading to release of ADP,” explains Muto. “I did not expect that residues in α-tubulin would play such an important role.”
In future studies, Muto and Uchimura hope to further dissect the amino acid network that communicates these structural changes across the kinesin protein. Since microtubules play a key role in diverse cellular functions beyond molecular transport, Muto believes that their mutational analysis strategy should also offer a powerful tool for studying processes ranging from the separation of chromosome pairs during cell division to cilia-mediated cell propulsion.
References
- 1. Uchimura, S., Oguchi, Y., Hachikubo, Y., Ishiwata, S. & Muto, E. Key residues on microtubule responsible for activation of kinesin ATPase. The EMBO Journal 29, 1167–1175 (2010). doi: 10.1038/emboj.2010.25
