Jun. 11, 2010 Research Highlight Chemistry
Projecting pain relief
Radioactively labeled drugs can track inflammation in the brain
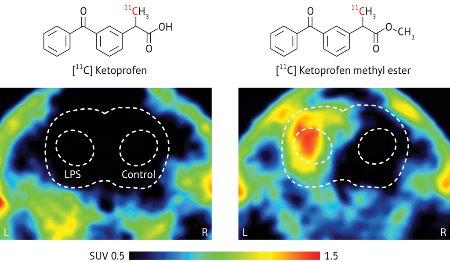 Figure 1: PET images of an inflamed rat brain summated from 5 to 45 minutes after administration of [11C]Ketoprofen (left) and [11C]Ketoprofen methyl ester (right). The inflammation results from the injection of lipopolysaccharide (LPS) into the left, inner part of the brain. Reproduced, with permission, from Ref. 1 © 2010 Wiley-VCH Verlag GmbH & Co. KGaA
Figure 1: PET images of an inflamed rat brain summated from 5 to 45 minutes after administration of [11C]Ketoprofen (left) and [11C]Ketoprofen methyl ester (right). The inflammation results from the injection of lipopolysaccharide (LPS) into the left, inner part of the brain. Reproduced, with permission, from Ref. 1 © 2010 Wiley-VCH Verlag GmbH & Co. KGaA
Widely prescribed, nonsteriodal anti-inflammatory drugs (NSAIDs), such as Ibuprofen and Ketoprofen, inhibit enzymes called cyclooxygenases (COXs) that regulate pain, fever and other inflammations in the body. Now, a specially synthesized series of NSAIDs, and their so-called ‘2-arylpropionic acid derivatives’ labeled with carbon-11 (11C) radioisotope, can be used to trace inflammations in vivo using the bioimaging technique positron emission tomography (PET)1. This development results from a research team led by Masaaki Suzuki at the RIKEN Center for Molecular Imaging Science, Kobe.
Many researchers have designed radioisotope-labeled COX inhibitors to target and monitor COXs in living systems. Most efforts have focused on COX-2, a COX enzyme that can serve as a biomarker for early disease diagnosis because its concentration increases rapidly in cells during inflammation. However, no one has managed to image this enzyme using radioisotope labels to date.
Suzuki and his team created their radiotracer library through a general and efficient approach. First, they reacted each NSAID precursor with a small 11C-containing molecule for two minutes to produce the NSAID methyl esters by [11C]carbon–carbon bond formation. Then, they heated the esters at 50 °C under basic conditions—the pH of the solution was greater than 9—for one minute to generate the 11C-labeled drugs.
According to Suzuki, the short half-life of 11C expedites the screening of 11C-labeled compounds and makes their biological function easier to evaluate. “In addition, the [11C]carbon–carbon bond is metabolically very strong, providing highly reliable PET images and ideal molecular probes for investigations of dynamic behavior in vital in vivo systems,” he says.
After injecting a toxic bacteria-derived substance known as lipopolysaccharide into the left, inner-part of rat brains to induce inflammation, the researchers administrated intravenously the 11C-labeled compounds for evaluation. They discovered that the radioactivity of [11C]Ketoprofen methyl ester was highly concentrated in the inflamed area, but that of [11C]Ketoprofen was not (Fig. 1). These PET experiments indicated that the methyl ester readily penetrated the blood–brain barrier—a thin layer of blood vessel cells that separates circulating blood from cerebrospinal fluid and prevents particular molecules from entering the brain—and delivered the drug to the inflammation upon hydrolysis.
The researchers are planning to label NSAIDs with fluorine-18 that has a longer half-life. “The information obtained from longer times will give a more accurate and detailed analysis of dynamic behaviours in vivo,” says Suzuki. “We also hope to extend this research on brain inflammation to other important diseases by developing novel optimized PET probes.”
References
- 1. Takashima-Hirano, M., Shukuri, M., Takashima, T., Goto, M., Wada, Y., Watanabe, Y., Onoe, H., Doi, H. & Suzuki, M. General method for the 11C-labeling of 2-arylpropionic acids and their esters: construction of a PET tracer library for a study of biological events involved in COXs expression. Chemistry — A European Journal 16, 4250–4258 (2010). doi: 10.1002/chem.200903044
