Aug. 3, 2012 Research Highlight Biology
Unsung heroes of antibody production
The discovery of a cell-specific regulatory mechanism helps overturn a long-standing model of immune system function
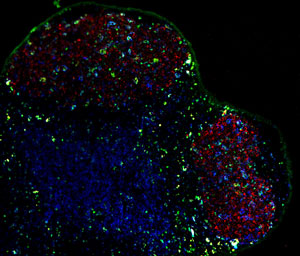 Figure 1: IL-4-secreting Tfh cells (green) reside alongside the B cells of the germinal center (red), and enable them to produce a full spectrum of antibody subtypes. © 2012 Masato Kubo, RIKEN Research Center for Allergy and Immunology
Figure 1: IL-4-secreting Tfh cells (green) reside alongside the B cells of the germinal center (red), and enable them to produce a full spectrum of antibody subtypes. © 2012 Masato Kubo, RIKEN Research Center for Allergy and Immunology
B cells are the body’s antibody factories, standing by to churn out molecules that selectively target foreign threats as a component of the humoral immune response. However, this process also requires T cells to secrete a protein known as interleukin-4 (IL-4), which promotes a mechanism called ‘class switching’ that enables production of functionally specialized antibody subtypes.
It remains unclear exactly which T cells generate the IL-4 signal. Although many scientists favor the Th2 subclass of helper T cells, there are strong arguments against this model as well. “This subset of cells cannot get into the follicles where these B cells are located,” explains Masato Kubo of the RIKEN Research Center for Allergy and Immunology, Yokohama. “Nevertheless, most textbooks say that Th2 cells control the antibody response.”
Kubo and colleagues have now provided strong evidence that a recently discovered class of follicular T (Tfh) cells generates the IL-4 signal that kicks B cells into high gear1. In a previous study, his group identified several stretches of DNA within the gene encoding IL-4 that might regulate its activity2. One candidate sequence, CNS2, had little effect on Th2 production of IL-4, but mice lacking this genomic region nevertheless showed severe defects in class-switching. “We started thinking other T cell subsets might be responsible for IL-4-mediated humoral immune responses,” he says.
By placing a fluorescent gene under the control of CNS2, the researchers showed that this regulatory region is specifically active in Tfh cells. As their name suggests, Tfh cells reside within follicles, in close proximity to the germinal centers where B cells mature. They also actively secrete IL-4 (Fig. 1), albeit by an apparently distinct mechanism from Th2 cells. Kubo’s team found that mutant mice lacking CNS2 can produce mature Tfh cells, but these cells expressed greatly reduced levels of IL-4. By comparison, Th2 cells were only minimally affected. Nevertheless, these genetically modified mice showed striking deficits in their production of several antibody subclasses, including immunoglobulin E (IgE) and G1 (IgG1).
These findings proved surprising at multiple levels. “IL-4 is a critical cytokine for controlling IgG1 and IgE antibody responses, but its expression in Th2 and Tfh cells is independently regulated by distinct elements,” says Kubo. “Furthermore, Tfh and not Th2 cells are the T cell subset responsible for Th2-type humoral immune responses.” He and his colleagues are working to identify the factors that act on CNS2, which could ultimately offer useful drug targets for controlling autoimmune disease by restraining antibody production.
References
- 1. Harada, Y., Tanaka, S., Motomura, Y., Harada, Y., Ohno, S., Ohno, S., Yanagi, Y., Inoue, H. & Kubo, M. The 3’ enhancer CNS2 is a critical regulator of interleukin-4-mediated humoral immunity in follicular helper T cells. Immunity 36, 188–200 (2012). doi: 10.1016/j.immuni.2012.02.002
- 2. Tanaka, S., Motomura, Y., Suzuki, Y., Yagi, R., Inoue, H., Miyatake, S. & Kubo, M. The enhancer HS2 critically regulates GATA-3-mediated Il4 transcription in TH2 cells. Nature Immunology 12, 77–85 (2011). doi: 10.1038/ni.1966
