Nov. 7, 2014 Research Highlight Biology
Bacteria and immune cells forge a productive partnership
Immune cells act as essential intermediaries between the intestines and ‘friendly’ gut bacteria in the effort to prevent infection
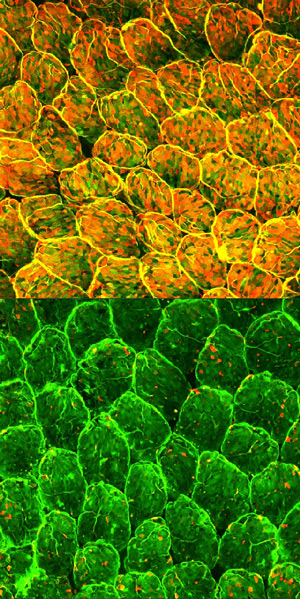 Figure 1: Normal mouse intestinal epithelial cells show pervasive fucosylation (top). Selective depletion of immune cells known as type 3 innate lymphoid cells dramatically suppresses fucosylation (bottom). Reproduced from Ref. 1 © 2014 Y. Goto et al.
Figure 1: Normal mouse intestinal epithelial cells show pervasive fucosylation (top). Selective depletion of immune cells known as type 3 innate lymphoid cells dramatically suppresses fucosylation (bottom). Reproduced from Ref. 1 © 2014 Y. Goto et al.
To prevent infection, the intestinal wall relies on the support of its own ‘home-grown’ army of commensal bacteria. These gut microbiota collaborate with and are in turn regulated by their host’s immune system via a variety of mechanisms. As part of a research team led by Hiroshi Kiyono from the University of Tokyo, Mitsuo Sakamoto, Yoshiyuki Goto and colleagues from the RIKEN BioResource Center have now helped to illuminate one mechanism by which the gut microbiota and immune cells collaborate1.
The study began with the discovery of a population of epithelial cells in the intestinal lining that become decorated with the sugar fucose. “Previous reports have shown that epithelial fucose is essential for host–microbiota interaction,” says Sakamoto. “These data prompted us to identify the mechanism that induces this epithelial fucosylation.”
The evidence to date has suggested that interaction with certain commensal bacteria stimulates this fucosylation directly. The researchers verified that mice entirely lacking gut microbiota have far fewer fucosylated epithelial cells, and, in particular, that a subset of microbes known as segmented filamentous bacteria (SFB) appear to make a major contribution to this process. However, the SFB cannot achieve this fucosylation on their own. The team found that these bacteria must collaborate with immune cells known as type 3 innate lymphoid cells (ILC3s), which serve as a front-line defense against infection.
The researchers subsequently determined that interaction with SFB and certain other commensal species causes ILC3s to release a signaling factor that stimulates epithelial production of an enzyme responsible for fucosylation. Interestingly, they also uncovered that a microbiota-independent molecule produced by ILC3s functions cooperatively with the microbiota-dependent signaling factor to induce epithelial fucosylation.
Together, these processes contribute to the formation of a fucose layer on the surface of the intestinal epithelia that effectively wards off infection. Mice lacking the primary fucosylation enzyme induced by ILC3s displayed suppressed fucosylation (Fig. 1) and proved to be far more susceptible to severe infection with Salmonella typhimurium, possibly because the pathogenic bacteria find it easier to attach to the exposed intestinal lining.
The findings could be relevant to a wide variety of disease states, and Sakamoto intends to explore this possibility in future studies. “It will be important to identify the specific bacteria that recognize epithelial fucose and how these bacteria affect the development of diseases such as inflammatory bowel disease,” he says. “More fundamentally, it will also be important to examine whether the mechanism we showed here is directly applicable in humans.”
References
- 1. Goto, Y., Obata, T., Kunisawa, J., Sato, S., Ivanov, I. I., Lamichhane, A., Takeyama, N., Kamioka, M., Sakamoto, M., Matsuki, T. et al. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science 345, 1254009 (2014). doi: 10.1126/science.1254009
