Nov. 28, 2014 Research Highlight Biology
An inner look at the inner ear
The earliest stages of ear development involve a localized signaling cascade
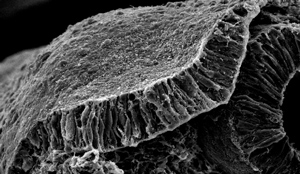 Figure 1: Invagination of the otic placode (shown) during embryonic development involves a localized signaling cascade that causes the tissue to thicken and then bend and pinch. © 2014 Raj Ladher, RIKEN Center for Developmental Biology
Figure 1: Invagination of the otic placode (shown) during embryonic development involves a localized signaling cascade that causes the tissue to thicken and then bend and pinch. © 2014 Raj Ladher, RIKEN Center for Developmental Biology
The proteins associated with driving the cell shape changes that internalize the embryonic inner ear have been identified by Raj Ladher and colleagues from the RIKEN Center for Developmental Biology1. “Our hope,” says Ladher, “is that by understanding the morphogenesis of the inner ear, clinicians will become more aware of what to look for in their diagnosis of otic developmental defects.”
During the earliest stages of embryonic development, the inner ear forms as a thickening of cells on the outside of the embryo. This cell mass, known as the otic placode (Fig. 1), is then internalized, or invaginated, in two stages: cells first expand at their base to create a small depression, then the tops of the cells constrict to progressively deepen the crater.
Both steps of this invagination process rely on a contractile protein called myosin-II that works in tandem with another protein called actin. Myosin-II breaks up strings of actin during the first, basal expansion phase, whereas the same protein triggers a tightening of the actin filaments in the second, apical constriction phase.
To determine the molecular cues involved in these contrasting activities of myosin-II, Ladher and his colleagues studied otic placode development in chicken embryos. The team showed that the Ras homolog gene family member A (RhoA) protein, which is known to regulate the actin cytoskeleton, had a localized activity pattern that facilitated apical constriction.
The researchers revealed a cascade of signaling proteins behind this process. A protein called Celsr1 was found to accumulate at the apical cell junctions and determine cell polarity. This protein in turn recruited the ArhGEF11 protein, which activated RhoA. In this way, RhoA expression—and thus actin contraction—was restricted to the apical surface.
Ladher notes that this kind of coordinated invagination of an epithelial layer often occurs in development. “So it is likely,” he says, “that this kind of epithelial remodeling could be mechanistically conserved in a number of systems, in particular during neural tube closure.” The neural tube is the embryo’s precursor to the central nervous system, and abnormal development in this critical organ leads to birth defects such as spina bifida. The invagination cascade found in the inner ear is almost identical to that found previously in the neural tube.
“If a common morphogenetic toolbox is used for many different epithelial shaping processes,” Ladher says, “it might be possible to adapt this research to offer therapies for neural tube defects, body wall closure defects and wound healing.”
References
- 1. Sai, X., Yonemura, S. & Ladher, R. K. Junctionally restricted RhoA activity is necessary for apical constriction during phase 2 inner ear placode invagination. Developmental Biology 394, 206–216 (2014). doi: 10.1016/j.ydbio.2014.08.022
