Aug. 14, 2015 Research Highlight Biology
Choosing sides in embryo development
The asymmetric expression of two genes assists developing mouse embryos in establishing their front and rear sides
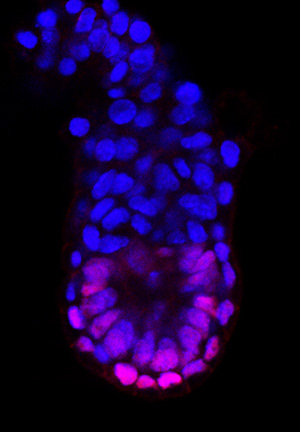 Figure 1: Five-and-a-half days after fertilization, asymmetric expression of the OTX2 protein (magenta) helps to establish the anterior and posterior ends of the mouse embryo (blue labeling indicates cell nuclei). © 2015 Go Shioi, RIKEN Center for Life Science Technologies
Figure 1: Five-and-a-half days after fertilization, asymmetric expression of the OTX2 protein (magenta) helps to establish the anterior and posterior ends of the mouse embryo (blue labeling indicates cell nuclei). © 2015 Go Shioi, RIKEN Center for Life Science Technologies
The gene expression changes that determine front (anterior) and rear (posterior) identity in the mouse embryo have been ascertained by RIKEN researchers1.
As an embryo develops from a simple sphere into a highly complex body plan, it establishes a biochemical ‘map’ to guide subsequent development. Variations in gene expression within different cell populations can designate the final orientation of the embryo, enabling anatomically appropriate development to occur.
In the mouse embryo, the gene expression changes that establish the anterior and posterior begin about five days after fertilization with the formation of tissue known as the distal visceral endoderm (DVE). The DVE subsequently migrates to form the anterior visceral endoderm (AVE), which defines the anterior end of the embryo. The molecular basis for this process is poorly understood.
“Our previous studies analyzed expression patterns of DVE and AVE genes,” explains co-author Go Shioi, now at the RIKEN Center for Life Science Technologies. “However, those analyses did not reveal details at an individual cell level.”
By exploiting various labeling strategies, the researchers, who were led by Shinichi Aizawa of the RIKEN Center for Developmental Biology, could monitor the shifting expression patterns of five genes expressed by AVE cells. Their analysis revealed that by six-and-a-half days after fertilization, the AVE is composed of four zones of cells, which are distinguishable by their differential expression levels of the five genes.
To identify what initiates this developmental pattern, Aizawa’s team examined embryos earlier in development. They discovered that none of the five genes was detectably active until five days after fertilization. After this point, three of the five genes exhibited symmetric expression patterns in the embryo, which suggests that they do not directly contribute to defining the anterior–posterior axis. In contrast, the expression of the other two genes, OTX2 and DKK1, was skewed toward what would ultimately become the posterior end of the embryo. AVE formation occurs when a subset of DVE cells migrates from this zone of elevated OTX2 and DKK1 expression (Fig. 1).
“This finding was interesting and exciting for us because we have been mostly focused on the functional analysis of Otx2 and Dkk1 rather than other DVE–AVE genes,” explains Shioi. Aizawa and his colleagues have identified numerous molecules that affect Otx2, which appears to be particularly critical in determining the anterior–posterior axis, but none of the molecules explain the asymmetric expression of OTX2. Thus, the hunt for novel Otx2 regulators will be a top priority moving forward.
References
- 1. Hoshino, H., Shioi, G. & Aizawa, S. AVE protein expression and visceral endoderm cell behavior during anterior–posterior axis formation in mouse embryos: Asymmetry in OTX2 and DKK1 expression. Developmental Biology 402, 175–191 (2015). doi: 10.1016/j.ydbio.2015.03.023
