Apr. 8, 2016 Special Feature Physics / Astronomy
RIKEN discovers superheavy element 113
Researchers at RIKEN will be the first in Asia to name an element in the periodic table
On New Year’s Eve 2015, a group of researchers at the RIKEN Nishina Center for Accelerator-Based Science received news of historical scientific importance to Japan and the rest of the world. The International Union of Pure and Applied Chemistry (IUPAC) would be giving RIKEN’s research group the honor of naming and determining the two-letter symbol for element 113. The news came more than 11 years after the RIKEN group, led by Kosuke Morita, first synthesized the element, and 3 years after they had conclusively demonstrated its decay chain. Element 113 will be the first element named by an Asian research institution.
Inside the atom
Superheavy elements exist for only a fraction of a second, which makes it very difficult to prove their existence. But studying these evasive atomic species is important for understanding the structure of the atomic nucleus, and could eventually lead to the discovery of the so-called island of stability, where elements with longer half-lives are predicted to exist.
Japan has a long tradition of research in nuclear physics, beginning at the turn of the 20th century with physicist Hantaro Nagaoka, who published an early model of the atom with electrons orbiting like Saturn’s rings around a central sphere. Several prominent researchers at RIKEN have made significant theoretical contributions to the field, including the “founder of modern physics in Japan”, Yoshio Nishina, as well as the first and second Japanese Nobel laureates, Hideki Yukawa and Shinichiro Tomonaga, respectively. On the experimental side, in 1937 RIKEN became home to Japan’s first cyclotron, which was an important pillar for nuclear physics research.
Over the years, RIKEN has developed the ability to design the world’s best-performing accelerators, thereby propelling itself to a leading global position in the field. Building on these achievements, RIKEN was able to not just discover element 113, but to also collect additional data on element 112, giving further credibility to the discovery claims of the GSI Helmholtz Center for Heavy Ion Research in Germany.
Hide and seek
 Kosuke Morita © 2016 RIKEN
Kosuke Morita © 2016 RIKEN
The search for element 113 began in September 2003, when Morita’s group used the RIKEN heavy-ion linear accelerator (RILAC) to fuse bismuth isotopes with zinc ions travelling at about 10 per cent of the speed of light, and then used the gas-filled recoil ion separator (GARIS) to identify the products. By smashing the two lighter elements together, they hoped to produce the heavier element 113.
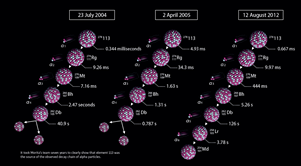
Morita succeeded in observing a signature of element 113 in 2004, and then again in 2005. The proof came in the form of alpha particles, composed of two protons and two neutrons, emitted as a heavy nucleus decays into a lighter one. Through his high-speed experiments, Morita was able to detect four alpha particles at times corresponding to element 113 decaying sequentially to dubnium-262 (element 105). The dubnium-262 atom then spontaneously split into many smaller fragments, a process known as fission. But neither these results nor the results produced by a separate group of researchers in Russia were enough to convince the IUPAC that the chain of alpha decays had been produced by element 113.
To form a better picture of the section of the decay chain from bohrium-266 (element 107) to lawrencium-258 (element 103), which had not been previously well characterized, the group performed a new experiment. They bombarded a curium target with a sodium beam to create bohrium-266 and its daughter nucleus, dubnium-262. With this demonstration, the grounds for a stronger claim were laid. The researchers just needed to wait to see element 113 decay past dubnium-262 through the alpha chain rather than through fission.
But it would be another seven years before they could observe the signature of element 113 once again. “I was not prepared to give up,” says Morita. “I believed that one day, if we persevered, luck would fall upon us again.”
Naming rights
 It took Morita’s team seven years to clearly show that element 113 was the source of the observed decay chain of alpha particles. © 2016 RIKEN
It took Morita’s team seven years to clearly show that element 113 was the source of the observed decay chain of alpha particles. © 2016 RIKEN
Finally, on 12 August 2012, Morita’s group measured a sequence of six alpha particles that clearly showed that element 113 was the source of the decay chain. This time, following the four initial decays, dubnium-262 continued to undergo two more alpha decays, transforming into lawrencium-258 and then mendelevium-254 (element 101). The results provided sufficient evidence for the IUPAC to recognize Morita’s group with the discovery of element 113, and to give them the privilege of naming it.
“We must also keep in mind that this achievement was made possible not by the Morita group alone, but also by a decade of effort by RIKEN’s Accelerator Group,” notes Hideto En’yo, director of the RIKEN Nishina Center for Accelerator-Based Science. Researchers and technicians at the Accelerator Group, in collaboration with industry, have managed to increase the beam intensity a thousand-fold compared to the first GARIS system built in 1987, and have made it possible to carry out experiments on a 24-hour basis.
“I am proud to know that Japan and RIKEN have produced something that will be added to the list of things that make up the Universe,” says RIKEN President Hiroshi Matsumoto. “The Japanese people share in this pride, and I hope the acknowledgement of Japan’s role in the discovery of element 113 will stimulate greater interest in science among people in general and children in particular.”
While the world focuses its attention on the new name that will be given to element 113, Morita is moving on to the next challenge. “We plan to look to the uncharted territory of element 119 and beyond, aiming to examine the chemical properties of the elements in the seventh and eighth rows of the periodic table, and someday to discover the island of stability,” he says.
References
- 1. Press release: It’s official! Element 113 was discovered at RIKEN
