Sep. 23, 2016 Research Highlight Biology
A protein pushes ahead of the curve
A cell-signaling enzyme coordinates the three-dimensional reorganization of cell membranes
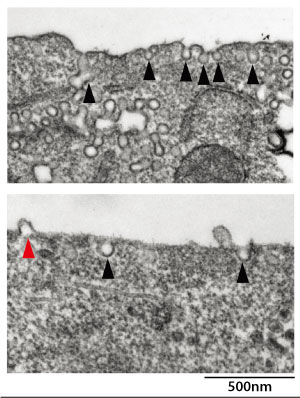 Figure 1: Electron microscopy images show that relative to normal cells (top), cells deficient in the protein phospholipase Cβ1 (bottom) produce considerably fewer caveolae (black arrowheads) and exhibit atypical membrane bulging (red arrowhead). Reproduced, with permission, from Ref. 1 © 2016 T. Inaba et al.
Figure 1: Electron microscopy images show that relative to normal cells (top), cells deficient in the protein phospholipase Cβ1 (bottom) produce considerably fewer caveolae (black arrowheads) and exhibit atypical membrane bulging (red arrowhead). Reproduced, with permission, from Ref. 1 © 2016 T. Inaba et al.
Exactly how cells ingest large molecules may be explained by the discovery of a new role for a known protein by RIKEN researchers1.
The scientists began by conducting a comprehensive search for proteins that can cause cell surfaces to curve, which is important because the shape of a cell is integral to its function. Curving proteins work by binding to the fatty molecules that make up the membrane, known as lipids. The more proteins attach to lipids, the greater the curvature of the cell surface.
The team combined membranes made of different types of lipids with proteins isolated from various mouse tissues and homed in on a protein that caused the lipid membranes to bend into tube-like structures.
To the researchers’ surprise, the isolated protein turned out to be phospholipase Cβ1 (PLCβ1), an enzyme found in the brain known to be involved in various cellular signaling processes, including those that drive cell division and maturation. Interacting with the cell membrane, however, the protein seemed to play a different role.
When coupled with a lipid called phosphatidylethanolamine (PE), PLCβ1 appeared to initiate the formation of indented structures in the cell membrane known as caveolae. These indents contribute to processes such as signaling or the internalization of biomolecules from outside the cell. Electron microscopy showed that cells with sharply reduced levels of PLCβ1 produced notably fewer caveolae, and those that did form, exhibited abnormal structures (Fig. 1).
That PE was involved also came as a surprise to Toshihide Kobayashi of the RIKEN Lipid Biology Laboratory and colleagues. “This is a well-conserved lipid, from bacteria to mammals,” says Kobayashi, “but previously, it was not known to be a molecule that PLCβ1 acts on.” Subsequent experiments showed that the protein and lipid interact via a structural element that bears a close resemblance to elements found in other curvature-inducing proteins.
PLCβ1 is best known as a producing enzyme of two signaling molecules called inositol 1-4-5-trisphosphate (IP3) and diacylglycerol (DAG) from phosphatidylinositol-4,5-bisphosphate (PIP2). But Kobayashi’s team determined that mutant versions of the protein lacking this enzymatic function were still able to bind to membranes and initiate tubule formation.
Kobayashi is currently trying to determine whether the enzyme’s activity might somehow still be involved in the membrane-bending process. One hypothesis is that it drastically alters the physical shape of lipids near PE by changing PIP2 to DAG. “This could induce a big alteration in the membrane curvature,” says Kobayashi. It is also likely that the enzyme’s activity does not play a role in PLCβ1-induced membrane curvature. Clarifying the precise contribution of PLCβ1 is a top priority for the team moving forward.
Related contents
- Wrapping up a cell biology riddle
- Unearthing the pathways of plasticity
- Helping neurons find their way
References
- 1. Inaba, T., Kishimoto, T., Murate, M., Tajima, T., Sakai, S., Abe, M., Makino, A., Tomishige, N., Ishitsuka, R., Ikeda, Y. et al. Phospholipase Cβ1 induces membrane tubulation and is involved in caveolae formation. Proceedings of the National Academy of Sciences USA 113, 7834–7839 (2016). doi: 10.1073/pnas.1603513113
