Sep. 1, 2013
Novel rhodamine luminophore
RIKEN No.: 07835
Inventors
Shinichiro Kamino and Shuichi Enomoto (Next-generation Imaging Team)
Background
The design and synthesis of high-potential luminophores is an attractive research topic in the field of molecular imaging technology and photonic and electronic devices. Rhodamine dyes are one of the most popular fluorescent dyes and are widely employed as important fluorescent materials for the advanced technology. However, rhodamine dyes exhibit a strong tendency to form aggregates when dissolved in concentrated solutions or doped on solids, which causes serious disadvantages, such as quenching and low photosensitivity, for their applications. Recently, luminophores possessing the property of aggregation-induced emission enhancement (AIEE) have attracted attention as promising materials for electroluminescent devices and optical sensing.
Summary
RIKEN researchers have developed novel rhodamine luminophore, 3’,3’’-bis(oxospiroisobenzofuran)-3,7-bis(dialkylamino)benzopyrano-xanthene derivatives (ABPX). ABPX has been synthesized by the condensation of individual benzophenone derivative with resorcinol. The emission behavior of ABPX was directly opposite to the aggregation-caused quenching (ACQ) of conventional rhodamine dyes. ABPX series exhibit unique AIEE.
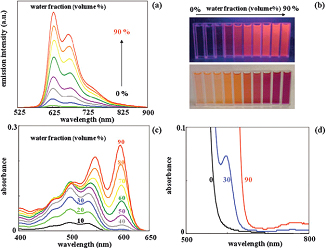
(a) Emission spectra of 500 μM of ABPX in water/THF mixtures by surface photometric method.
(b) Photograph of the solutions, top: under 365 nm irradiation, bottom: under room light.
(c) Absorption spectra of 5 μM of ABPX in water–THF mixtures.
(d) Level-off tails in 0%, 30%, and 90% water/THF mixtures owing to the Mie scattering effect.
Merits
- No quenching. High photosensitivity.
- Near-infrared region (NIR)
- "photo-switching" in the on–off luminescent states of AIEE
Applications
- Fluorescent probe
- Photosensitizers for imaging and photodynamic therapy
- Light-absorptive material for dye-sensitised solar cell
- Laser dyes
- Organic light emitting display
References
- 1.S. Kamino and S. Enomoto, US patent 8134017.
- 2.S. Kamino, et.al. Chem. Commun., 46, 9013 (2010).
- 3.S. Kamino, et.al. Phys. Chem. Chem. Phys.,15, 2131 (2013).
- 4.S. Kamino, et.al. Chem. Asian. J., 2013, doi: 10.1002/asia. 201300515
