Jul. 27, 2013
Real-time lithium-oxigen reaction process visualized for the first time
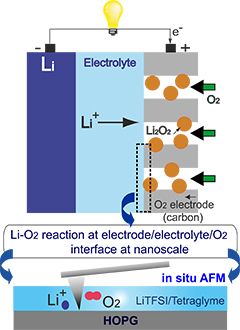
Researchers from the RIKEN Byon Initiative Research Unit have succeeded in visualizing the lithium-oxygen (Li−O2) electrochemical reaction that takes place in nonaqueous Li-O2 batteries, for the first time.
The researchers used a technique called operando atomic force microscopy (AFM), coupled with electrochemical equipment to observe the reaction process at the nano/micrometer scale. They used a highly oriented pyrolytic graphite (HOPG) electrode, an O2 gas-saturated lithium ion–containing tetraglyme, representative of the carbon cathode, and an ether–based electrolyte extensively used in Li–O2 batteries.
They observe the nucleation, growth, and decomposition of Li2O2 nanoplates, product of the Li-O2 reaction, formed following the oxygen reduction reaction.
Their observations provide the first visualization of the Li−O2 reaction process and reveal useful information about the reaction and its products, and will help scientists understand the reaction and design the optimum conditions for Li–O2 batteries.
The work is published in the Journal of the American Chemical Society DOI: 10.1021/ja405188g
