Dec. 25, 2009 Press Release Biology
New fluorescent proteins report electrical signals of brain cells
A new fluorescent protein has illuminated complex neural networks of the hippocampus. The protein was engineered by researchers at RIKEN to help analyze rapid electrical signals in populations of nerve cells and provides a unique window onto cellular-dynamics of neuronal webs. Further work with this protein is expected to dramatically extend the scope of research into brain function.
One of the key challenges in analyzing neural network dynamics is to monitor the activity of multiple neurons simultaneously. Voltage-sensitive fluorescent proteins (VSFP) make such analysis possible by encoding voltage sensors at the genetic level, enabling researchers to non-invasively target and visualize the activity of specific cell populations. VSFPs have, until now, suffered from interference with tissue background fluorescence and poor long-term expression in nerve cells.
A new series of red-shifted VSFPs, designed by a research team at the RIKEN Brain Science Institute, has overcome these limitations. By fusing the voltage-sensitive domain of a voltage-sensing phosphatase (Ci-VSP) to red-shifted fluorescent proteins, the researchers generated a series of VSFPs emitting different spectral colors. In a paper in the journal Chemistry & Biology, the researchers use these proteins to uncover details of the voltage-sensing mechanism in Ci-VSP, while also demonstrating the effectiveness of one variant (VSFP3.1_mOrange2) for analysis of electrical signals in hippocampal neurons.
The glimpse of the cellular-level dynamics of neuronal networks provided by VSFPs will vastly expand our understanding of information processing in the brain. By extending and clarifying the mechanisms of existing VSFPs, the new family of red-shifted proteins brings this potential one step closer to reality, enabling groundbreaking advances in understanding brain function.
Contact
Thomas Knöpfel
Laboratory for Neuronal Circuit Dynamics
RIKEN Brain Science Institute
Tel: +81-(0)48-467-9740 / Fax: +81-(0)48-467-9739
Jens Wilkinson
RIKEN Global Relations and Research Coordination Office
Tel: +81-(0)48-462-1225 / Fax: +81-(0)48-463-3687
Email: pr[at]riken.jp
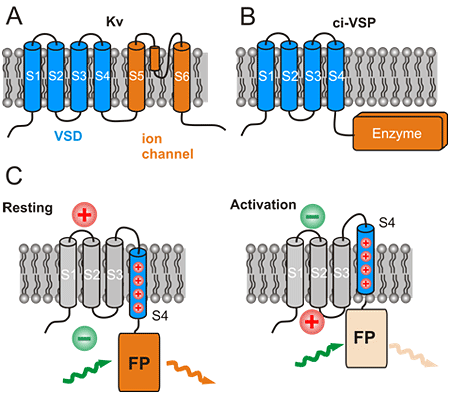
Figure 1: Structure and function of natural voltage sensing proteins and derivation of the design of engineered voltage sensitive fluorescent proteins (VSFPs)
(A) and (B) show the structure of voltage gated potassium channels (Kv) and voltage gated enzymes (ci-VSP). The number S1 to S6 (S4) indicate transmembrane segments. S1 to S4 constitute the voltage sensor domain (VSD). The VSD controls the opening of an ion channel or activation of an enzyme in Kv and ci-VSP, respectively. C: Control of a fluorescent protein (FP) brightness by the VSD in VSFPs. Activation of the membrane from rest (such as during activation of brain cells) moves the positively charged S4 segment which in turn moves the FP towards the membrane. The brightness of the FP is decreased in this new position. This is seen as a decreased orange colored fluorescence (orange colored light wave) with same green excitation light (green colored light wave).
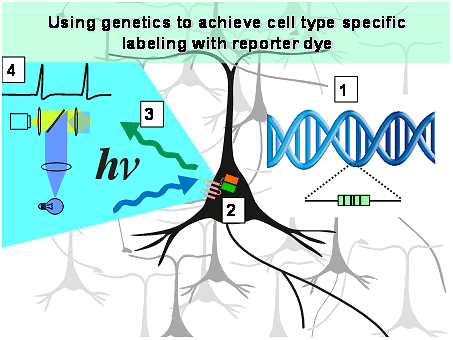
Figure 2: Genetically encoded voltage sensitive fluorescent proteins are encoded in DNA [1] which can be introduced into the genome of nerve cells.
The nerve cells then synthesized the protein [2], so that an optical imaging system [3] can be used to monitor the activity of these cells [4]. Using genetic methods, the fluorescent probe can be targeted to selected types of nerve cells.
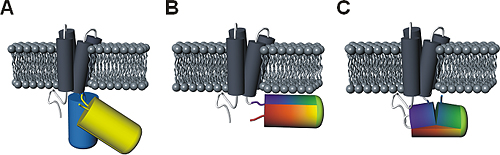
Figure 3: Comparison of principal designs for voltage-sensitive fluorescent proteins
(A) FRET-based voltage probe. Cyan- and yellow-emitting GFP variants are connected by a short linker.
(B) Single FP-based voltage probe.
(C) Circularly permuted FP-based voltage probe. The N- and C-terminal portions of GFP are swapped, resulting in new N- and C-terminal ends. The color gradient (from purple to red) shows the progression from the N- to the C-terminus of the original protein. Note the direct attachment of the GFP reporter to the S4 domain.
