Jun. 14, 2010 Press Release Biology Chemistry
New research uncovers elusive target of antifungal agent
A research team in Japan has uncovered the mechanism underlying the antifungal activity of theonellamide (TNM), a bioactive compound found in a species of marine sponge. The finding, reported in Nature Chemical Biology, provides new insights toward the development of antifungal drugs, with broader applications to drug analysis in areas such as cancer research.
Designing drugs that target fungal cells poses serious challenges due to their similarity to human cells at the molecular level. To avoid damaging human cells, such drugs exploit differences between mammalian and fungal cells, both of which, unlike bacteria, are in the same family of eukaryotes. The difficulty of this task results in toxic side effects and a shortage of effective antifungal agents.
The research team, headed by scientists at the RIKEN Advanced Science Institute, set out to tackle this problem by investigating the antifungal activity of theonellamides (TNMs), bicyclic peptides found in the marine sponge Theonella, on the fission yeast. Combining chemical-genetic profiling and biochemical and cellular analysis, they deduced that TNMs bind to a class of lipid molecules (3β-hydroxysterols) in the cell membrane to induce overproduction of 1,3-β-D-glucan, a component of the cell wall.
Antifungal activity in TNMs, the researchers showed, is thus different from other antifungals, which cause damage by impairing wall synthesis. Instead, TNMs damage fungal cells by abnormally promoting such synthesis, targeting not proteins as previously thought, but a class of lipids, including ergosterol. TNMs thus represent an entirely new class of sterol-binding molecules, offering a new direction in the development of antifungal agents and a powerful tool for drug analysis.
Contact
Minoru Yoshida
Chemical Genomics Research Group
RIKEN Advanced Science Institute
Tel: +81-(0)48-467-9516 / Fax: +81-(0)48-467-4676
Jens Wilkinson
RIKEN Global Relations and Research Coordination Office
Tel: +81-(0)48-462-1225 / Fax: +81-(0)48-463-3687
Email: pr@riken.jp
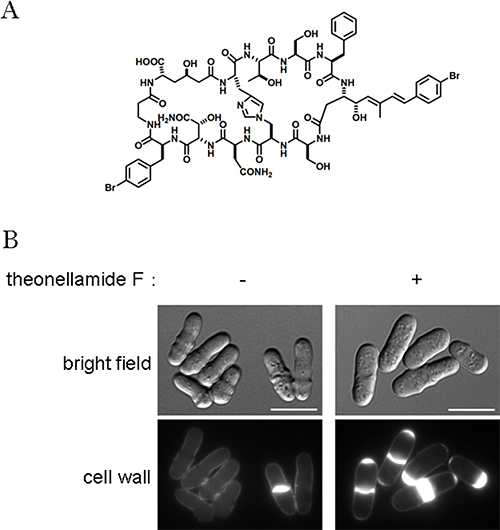
Chemical structure and cellular effect of theonellamide F
(A) Chemical structure of theonellamide F, one of the theonellamide family compounds.
(B) Abnormal cell wall synthesis induced by theonellamide F. In normal fission yeast cells, only septa are stained by calcofluor white, a fluorescent dye for detecting a major yeast cell wall component, 3-β-D-glucan (left panel). Upon theonellamide F treatment, 3-β-D-glucan is overproduced in septa and cell tips (right panel).
