Dec. 18, 2015 Press Release Biology
How excitatory/inhibitory balance is maintained in the brain
Just as a thermostat is used to maintain a balanced temperature in a home, different biological processes maintain the balance of almost everything in our bodies, from temperature and oxygen to hormone and blood sugar levels. In our brains, maintaining the balance—or homeostasis—between excitation and inhibition within neural circuits is important throughout our lives, and now, researchers at the RIKEN Brain Science Institute and Nagoya University in Japan, and École Normale Supérieure in France have discovered how disturbed inhibitory connections are restored. Published in Cell Reports, the work shows how inhibitory synapses are stabilized when the neurotransmitter glutamate triggers stored calcium to be released from the endoplasmic reticulum in neurons.
“Imbalances in excitation and inhibition in the brain has been linked to several disorders,” explains lead author Hiroko Bannai. “In particular, forms of epilepsy and even autism appear to be related to dysfunction in inhibitory connections.”
One of the key molecules that regulates excitation/inhibition balance in the brain is the inhibitory neurotransmitter GABA. When GABA binds to GABAA receptors on the outside of a neuron, it prevents that neuron from sending signals to other neurons. The strength of the inhibition can change depending on how these receptors are spaced in the neuron’s membrane.
While GABAA receptors are normally clustered together, continual neural activation of NMDA receptors by the neurotransmitter glutamate—as occurs naturally during learning and memory, or in epilepsy—leads to an excess of incoming calcium, which ultimately causes the receptors to become more spread out, reducing how much the neuron can be inhibited by GABA.
To combat this effect, the receptors are somehow continually re-clustered, which maintains the proper excitatory/inhibitory balance in the brain. To understand how this is accomplished, the team focused on another signaling pathway that also begins with glutamate, and is known to be important for brain development and the control of neuronal growth.
In this pathway glutamate binds to the mGluR receptor and leads to the release of calcium from internal storage into the neuron’s internal environment. Using quantum dot-single particle tracking, the team was able to show that after release, this calcium interacts with protein kinase C to promote clustering of GABAA receptors at the postsynaptic membrane—the place on a neuron that receives incoming neurotransmitters from connecting neurons.
These findings show that glutamate activates distinct receptors and patterns of calcium signaling for opposing control of inhibitory GABA synapses.
Notes Bannai, “it was surprising that the same neurotransmitter that triggers GABAA receptor dispersion from the synapse, also plays a completely opposite role in stabilizing GABAA receptors, and that the processes use different calcium signaling pathways. This shows how complex our bodies are, achieving multiple functions by maximizing a limited number of biological molecules.
Pre-activation of the cluster-forming pathway completely prevented the dispersion of GABAA receptors that normally results from massive excitatory input, as occurs in status epilepticus—a condition in which epileptic seizures follow one another without recover of consciousness. Bannai explains, “further study of the molecular mechanisms underlying the process we have uncovered could help develop treatments or preventative medication for pathological excitation-inhibition imbalances in the brain.
“The next step in understanding how balance is maintained in the brain is to investigate what controls which pathway is activated by glutamate. Most types of cells use calcium signals to achieve biological functions. On a more basic level, we believe that decoding these signals will help us understand a fundamental biological question: why and how are calcium signals involved in such a variety of biological phenomena?”
Reference
- Bannai H, Niwa F, Sherwood MW, Shrivastava AN, Arizono M, Miyamoto A, Sugiura K, Lévi S, Triller A, Mikoshiba K. Bidirectional control of synaptic GABAAR clustering by glutamate and calcium. Cell Reports. doi: 10.1016/j.celrep.2015.12.002
Contact
Laboratory Head
Katsuhiko Mikoshiba
Laboratory for Developmental Neurobiology
RIKEN Brain Science Institute
École Normale Supérieure, France
Email: triller@biologie.ens.fr Adam Phillips
RIKEN Global Relations and Research Coordination Office
Tel: +81-(0)48-462-1225 / Fax: +81-(0)48-463-3687
Email: pr@riken.jp
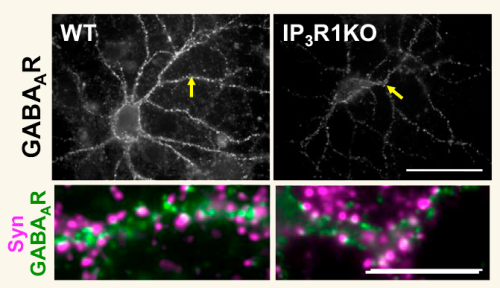
Knocking out the IP3 1 receptor in hippocampal neurons causes a reduction in GABAA receptor clustering at post-synaptic membranes
Left panels: Wild type; Right panels: IP31R Knockout; GABAA receptors (γ2 subunits) are stained in green. Note the reduction in green clusters in the KO mice.
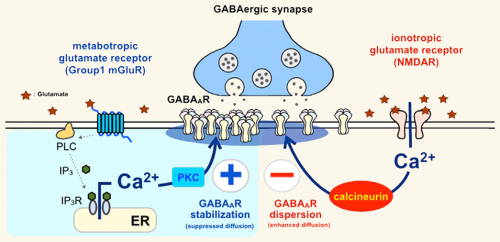
Schematic depicting the two opposing signaling pathways for modulating GABAA receptor positioning and thus the excitatory/inhibitory balance within the brain
Note that both pathways are triggered by glutamate and require calcium, although the calcium comes form different, non-overlapping sources.
