Dec. 31, 2015 Press Release Physics / Astronomy
It’s official! Element 113 was discovered at RIKEN
Element 113, discovered by a RIKEN group led by Kosuke Morita, has become the first element on the periodic table found in Asia. Rewarding nearly a decade of painstaking work by Morita’s group, a Joint Working Party of the International Union of Pure and Applied Chemistry (IUPAC) and International Union of Pure and Applied Physics (IUPAP) has recommended that the group, from the RIKEN Nishina Center for Accelerator-based Science (RNC), be given recognition for the discovery of the new element. This news was conveyed to Dr. Morita through a letter on December 31 from IUPAC.
In the late 1980s, the group began using RIKEN’s Linear Accelerator Facility and the GARIS ion separator, developed by Morita and his group, to explore new synthetic superheavy elements. The work of discovering new superheavy elements is very difficult, and the elements tend to decay extremely quickly—the isotopes of 113 produced at RIKEN lasted for less than a thousandth of a second. Researchers persevere, however, as the research is important for understanding the structure of atomic nuclei. Scientists hope that the work will lead eventually to the discovery of a so-called “island of stability” where elements with longer half-lives will be found.
The search at RIKEN for element 113 started in September 2003, when Morita’s group began bombarding a thin layer of bismuth with zinc ions travelling at about 10% the speed of light. Theoretically, they would occasionally fuse, forming an atom of element 113.
The team achieved its first success on July 23, 2004, less than a year after starting the experiment. Two atomic nuclei fused, leading to the creation of a nucleus of element 113, which quickly underwent four alpha decays to transform into dubnium-262 (element 105), which then underwent spontaneous fission. Less than a year later, on April 2, 2005, the team saw a second event—an identical decay to dubnium-262 followed by fission. Though these were good demonstrations, they were not considered conclusive evidence for the existence of element 113, because the decay chain did not demonstrate “firm connections to known nuclides” (according to the Joint Working Party’s 2011 report). The team pushed on with its efforts. In order to create a better picture of the decay chain from bohrium-266 to lawrencium-258, which had not been well characterized, the group performed a new experiment, where a sodium beam was collided with a curium target, creating borhium-266 and its daughter nucleus, dubnium-262. With this demonstration, the grounds for a stronger claim were laid. They just needed to wait to see an atom decaying through the alpha chain rather than spontaneous fission.Following the two initial events, however, the team’s luck seemed to run dry. "For over seven years,” says Morita, “we continued to search for data conclusively identifying element 113, but we just never saw another event. I was not prepared to give up, however, as I believed that one day, if we persevered, luck would fall upon us again.”
Then, on August 12, 2012, the group observed the crucial third event. This time, following the four initial decays, the dubnium-262 continued to undergo alpha decays rather than spontaneous fission, transforming into lawrencium-258 (element 103) and then finally mendelevium-254 (element 101). As the chain had been clearly characterized, it demonstrated clearly that element 113 was the source of the decay chain.In response to the new event, coupled with the group’s demonstration of the decay chain, IUPAC has announced that Morita’s group will be given priority for the discovery of the new element, a privilege that includes the right to propose a name for it.
For Morita, then, part of the coming year will be devoted to thinking of and proposing a formal name for element 113, but he is also looking forward to the next step in his research. “Now that we have conclusively demonstrated the existence of element 113,” he says, “we plan to look to the uncharted territory of element 119 and beyond, aiming to examine the chemical properties of the elements in the seventh and eighth rows of the periodic table, and someday to discover the island of stability."
The results of these experiments were reported in the Journal of Physical Society of Japan. The IUPAC report granting the naming rights to Morita's group will be published in an early 2016 issue of the IUPAC journal Pure and Applied Chemistry (PAC).
Comments
- Comment by Group Director Kosuke Morita
- Comment by RIKEN President Hiroshi Matsumoto
- Comment by RIKEN Nishina Center for Accelerator-Based Science Director Hideto En'yo
Reference
Papers reporting on the results of the experiments from RIKEN:
Experiment on the Synthesis of Element 113 in the Reaction209Bi(70Zn,n)278113
Journal of the Physical Society of Japan, Vol. 73, No. 10, October, 2004, pp. 2593–2596
DOI: 10.1143/JPSJ.73.2593Observation of Second Decay Chain from278113
Journal of the Physical Society of Japan Vol. 76, No. 4, April, 2007, 045001
DOI: 10.1143/JPSJ.76.045001Decay Properties of266Bh and262Db Produced in the 248Cm +23Na Reaction
Journal of the Physical Society of Japan, Vol. 78, No. 6, June, 2009, 064201
DOI: 10.1143/JPSJ.78.064201New Result in the Production and Decay of an Isotope,278113, of the 113th Element
Journal of the Physical Society of Japan 81 (2012) 103201
DOI: 10.1143/JPSJ.81.103201The report will be published based on the work published in those papers:
Paul J. Karol, Robert C. Barber, Bradley M. Sherrill, Emanuele Vardaci, and Toshimitsu Yamazaki, "Discovery of the elements with atomic numbers Z = 113, 115 and 117 (TBA)", To appear in Pure and Applied Chemistry, early 2016 issue.
Contact
Group Director
Kosuke Morita
Research Group for Superheavy Element
RIKEN Nishina Center for Accelerator-Based Science
Jens Wilkinson
RIKEN Global Relations and Research Coordination Office
Tel: +81-(0)48-462-1225 / Fax: +81-(0)48-463-3687
Email: pr@riken.jp
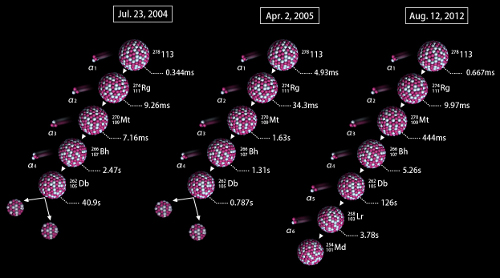
The decay routes seen in the three events
