Feb. 26, 2019 Press Release Biology
Scientists lay foundation for single-cell level understanding of DNA replication
In all living organisms, proliferating cells go through the fundamental process of DNA replication. Through this process, they faithfully duplicate their DNA and divide into two daughter cells. In eukaryotes, the genomic DNA is replicated during the S-phase of the cell cycle, and the order of the replication — DNA replication timing — is known to be strongly correlated with the higher-order structure of chromatin — the structure through which DNA is folded. Hence, studying DNA replication is important for understanding not only the faithful maintenance of the genome but also its higher-order structure.
In order to uncover the mechanisms that allow the stable maintenance of genomic DNA through cell divisions and the appropriate control of chromatin conformation, it is imperative to have a precise understanding of the DNA replication process at the single-cell level. However, little was known about the process of genome replication in single cells, because conventional biochemical methods required at least tens of thousands of cells to comprehensively investigate the process of DNA replication. How DNA replication proceeds in individual cells has remained a longstanding question in molecular biology.
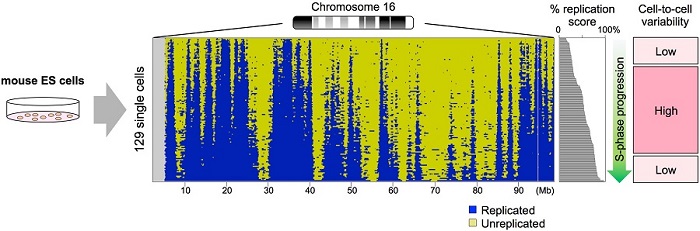
To solve this problem, a research team led by Ichiro Hiratani at the RIKEN Center for Biosystems Dynamics Research (BDR) and Shin-ichiro Takebayashi of Mie University, in collaboration with Osaka University, established a novel method to scrutinize DNA replication in individual cells. To perform the analyses at the single-cell level, they isolated genomic DNA from single cells in the S-phase—the phase of the cell cycle when DNA is replicated. They performed whole-genome sequencing, detecting the difference in copy number between each region of the genome. This method, which they named "scRepli-seq” (“single-cell DNA replication sequencing”), allowed them to obtain a detailed genome-wide view of replicated and unreplicated sequence distribution in each cell. They also succeeded in discriminating paternally- and maternally-derived homologous chromosomes in each cell by utilizing single-nucleotide variations between parents, making it possible to successfully visualize how each chromosome in the cell is replicated.
Analyses using mouse embryonic stem (ES) cells led to the finding that the variations in the DNA replication profiles were much smaller than expected, and that the genome replication process was highly conserved among cells. In addition, although the DNA replication profiles changed upon ES cell differentiation, cell-to-cell variations were small even after differentiation. Homologous chromosomes showed similar profiles within cells and were also similar between cells. In other words, the textbook view of DNA replication regulation based on averaged images of tens of thousands of cells turned out to be quite close to that found in individual cells.
Next, the researchers looked at the correlation between replication timing and higher-order structure of chromatin at the single-cell level. Recent studies have revealed that in the interphase cell nucleus, mammalian chromosomes are partitioned into megabase-sized self-associating units called topologically associating domains (TADs), which can be in either A (active) or B (inactive) subnuclear compartments. The research team found that single-cell DNA replication profiles reflected the A/B compartment organization very well. Because single-cell replication profiles were stable from cell to cell, this suggested that the A/B compartment structure may also be conserved from cell to cell.
While the DNA replication profiles of individual cells in a population were similar overall, the research team found that some genomic regions showed relatively large variability between cells. The first obvious factor was the time spent in the S-phase, with the earliest and the latest replicating sequences in S-phase exhibiting the least cell-to-cell variability. In contrast, the degree of variability became higher and more variable during the middle of the S-phase. Second, they found a relatively large cell-to-cell replication timing variability of developmentally-regulated sequences in ES cells. That is, sequences that undergo replication timing changes later in development showed higher replication timing variability in undifferentiated ES cells. This suggests an interesting possibility that their inherent replication timing instability, which probably reflects their compartmentalization instability, may confer competence for developmental regulation.
This study, along with a recent report (Dileep and Gilbert, Nat Commun2018), significantly improves our single-cell level understanding of DNA replication regulation and provides insights into how the higher-order structure of chromatin is regulated. Since the scRepli-seq method is simple and highly versatile, it can be applied to various organisms and has the potential to make a major contribution to research on DNA replication and genome regulation. According to Hiratani and Takebayashi, “It is almost certain that our methodology will have a broad impact on the basic biology of DNA replication, genome structure and instability, but it could also potentially serve as a comprehensive biomarker for human health and disease states, which could lead to new lines of research on various human diseases such as cancer.”
Reference
- Saori Takahashi, Hisashi Miura, Takahiro Shibata, Koji Nagao, Katsuzumi Okumura, Masato Ogata, Chikashi Obuse, Shin-ichiro Takebayashi, Ichiro Hiratani, "Genome-wide stability of the DNA replication program in single mammalian cells", Nature Genetics, 10.1038/s41588-019-0347-5
Contact
Team Leader
Ichiro Hiratani
Laboratory for Developmental Epigenetics
RIKEN Center for Biosystems Dynamics Research
Masataka Sasabe
RIKEN International Affairs Division
Tel: +81-(0)48-462-1225 / Fax: +81-(0)48-463-3687
Email: pr@riken.jp
Single-cell DNA replication profiles of mouse embryonic stem cells
