Sep. 28, 2021 Feature Highlight Biology
Moms risk life and limb for their young. WHY?
RIKEN researchers have identified the brain cells responsible for risk-taking parenting behavior in mice.
 A mother mouse and the pubs © anyaivanova / Shutterstock
A mother mouse and the pubs © anyaivanova / Shutterstock
It is often said that a mother will do anything for her children, even if it means risking her own life. Remarkably, this protective instinct is observed in all mammalian mothers, regardless of species.
Hormones involved in female reproduction, such as estrogen, are known to facilitate maternal motivation to care for their young. But the specific population of neurons that receives this hormonal information and affects maternal behavior has remained murky—until now. RIKEN researchers have recently identified specific brain cells involved in maternal care. The neurons they identified in mice contain a special protein called the calcitonin receptor, which, when silenced, drastically affects a mother’s nurturing abilities1.
“All mammalian parents, especially mothers, are highly motivated to sustain the life of their offspring,” says Kumi Kuroda at the RIKEN Center for Brain Science (CBS), who led the study. When cared for by their parents, baby animals have a higher chance at surviving in the wild, while human infants demonstrate better developmental outcomes.
“But in life there are so many difficulties and sometimes parents lose this motivation and abandon their infants,” she says. “So we wanted to find out what is happening when parental motivation is sustained even under difficult conditions. And to do that, we needed to identify the neural mechanisms involved in care,” says Kuroda, whose training as a psychiatrist inspired her to delve deeper into how the parental brain works.
A telling biomarker
For the past decade, Kuroda’s team has been studying the parent–infant bond in mammals. A few years ago, they discovered that the part of the brain responsible for nurturing behavior is the central medial preoptic area (cMPOA), which is located next to the hypothalamus just above the brainstem.
The team then explored further. The cMPOA is anatomically complex, containing more than seven different types of neurons. “We wanted to pin down which particular neuron is involved in the regulation of parental behavior,” explains Kuroda.
To do this, her team’s first task was to identify a biomarker that could reliably indicate which cMPOA neurons were active during nurturing behavior. From more than 20 candidate molecules, they identified one: a protein called the calcitonin receptor.
The receptor is found throughout the body and as its name suggests, usually binds to a hormone called calcitonin. “Its most famous function is calcium regulation in bone,” says Kuroda. However, calcitonin isn’t found in the brain and a different hormone—called amylin—binds to the receptor instead.
Upon further testing, Kuroda and her team discovered that postpartum mother mice had a higher number of cMPOA neurons expressing the calcitonin receptor compared with virgin females, males or fathers. These neurons also express estrogen receptor, and can mediate parturition-induced facilitation of maternal motivation.
“In mothers, the expression of the calcitonin receptor is eight times higher,” says Kuroda. “It is upregulated in postpartum mice and heightens maternal motivation to care for their young, even under risky conditions.”
More than just a marker
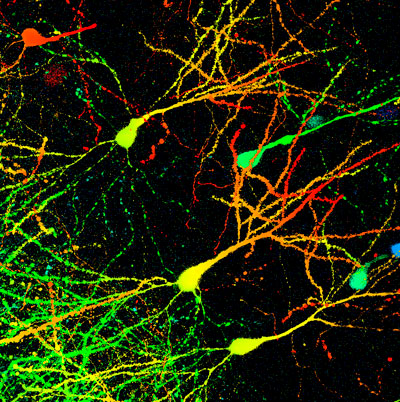 Mouse neurons. Kumi Kuroda's team have identified mouse neurons containing the calcitonin receptor protein that appear to affect the nurturing behavior of mouse mothers. When silenced, mother mice became less likely to nestle with their young or to take risks to protect them. © 2021 RIKEN; DR GOPAL MURTI / SCIENCE PHOTO LIBRARY
Mouse neurons. Kumi Kuroda's team have identified mouse neurons containing the calcitonin receptor protein that appear to affect the nurturing behavior of mouse mothers. When silenced, mother mice became less likely to nestle with their young or to take risks to protect them. © 2021 RIKEN; DR GOPAL MURTI / SCIENCE PHOTO LIBRARY
After identifying the calcitonin receptor as a marker for active neurons, the researchers then wondered: does the receptor itself play a role in enhancing nurturing behavior? “We wanted to see if the receptor was necessary for parental behaviors, and not just activated during nurturing,” explains Kuroda.
To explore this question, the team first genetically engineered mice (called Cre-transgenic), in order to specifically modify the calcitonin-receptor expressing neurons. The researchers then conducted two experiments.
In the first, they found that blocking the function of the calcitonin-receptor-expressing cMPOA neurons did not affect the ability of female mice to mate, become pregnant or deliver healthy pups. But what did change was the nurturing instincts of the mother mice: instead of gathering pups and grouping them together in a nest for warmth and feeding, they left them scattered throughout the cage. As a result, 83% of pups died the day after their birth, compared with 23.4% in the regular cMPOA group.
For the second experiment, Kuroda and her team devised an experiment involving a raised cross-shaped platform. The adult test mouse was placed on an enclosed (safe) arm of the cross, while mice pups were positioned on the three open arms, which were considered unsafe and scary for the mouse. The aim was to see if an adult mouse (either a postpartum mother or a virgin female) would venture out onto the open arms to rescue young.
“It seems a pretty scary task for mice,” says Kuroda. The platform is narrow and the mice have to pivot when they reach the edge containing the pup. “Sometimes their hind legs also drop down—it’s a 40 centimeter drop. In human terms, it’s like a two- to three-storey building.”
The bright lights of the lab made the whole scenario even more stressful, she says. “They’re already quite frightened and don’t want to go out.”
“But because mothers are highly motivated to care for their young, they will take the risk and save the pups on the high platform,” says Kuroda. However, mother mice with knocked-out calcitonin receptor genes exhibited measurable delays in rescuing the pups, and most virgin females in the experiment refused to do so.
“The conclusion is that the calcitonin receptor is not just a marker for nurturing-required neurons, but itself plays a role in the stimulation of postpartum maternal motivation, especially under risky conditions,” she says.
Kuroda’s team has now extended their studies to mammals other than mice—specifically, a species of long-tailed monkeys called marmosets.
“Examining the calcitonin-receptor-expressing cMPOA neuron’s role in these non-human primates should be more similar to what happens in humans,” says Kuroda. Her team has already begun work and are aiming to publish a paper in the near future.
Ultimately, Kuroda hopes her work will help children at risk of abuse and their parents. “The early experience children have with their parents directly affects their mental health and attitude towards others,” she says. “To prevent child abuse, we have to supportively look at parents’ behavior.”
She adds: “That’s why we really want to understand the brain mechanism for parental behavior.”
Reference
- 1. Yoshihara, C., Tokita, K., Maruyama, T., Kaneko, M., Tsuneoka, Y., Fukumitsu, K., Miyazawa, E., Shinozuka, K., Huang, A. J., Nishimori, K. et al. Calcitonin receptor signaling in the medial preoptic area enables risk-taking maternal care. Cell Reports 35, 109204 (2021). doi: 10.1016/j.celrep.2021.109204
About the researcher
Kumi Kuroda

Kumi Kuroda leads the Laboratory for Affiliative Social Behavior at the RIKEN Center for Brain Science. She received her Ph.D. in molecular biology from Osaka University's School of Medicine in 2002. She was a Human Frontier Science Program long-term fellow at McGill University in Canada from 2002−4, a Special Postdoctoral Researcher at the RIKEN Brain Science Institute from 2004−7, and was appointed as a laboratory head at RIKEN in 2008. Her lab has been studying the brain mechanisms of parent-infant relations in mice, monkeys and humans. Her team discovered that the central medial preoptic area in the basal forebrain is responsible for parental care and identified the 'transport response', in which infants are calmed by being carried by their parents. Her goal is to contribute to evidence-based support for healthy families.
