Jul. 5, 2006 Research Highlight Biology Medicine / Disease
How proteins work to make us move
Researchers begin to tackle the molecular basis of muscle function
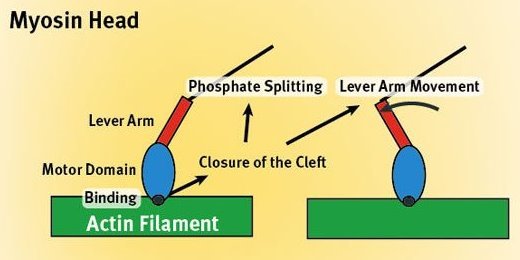 Figure 1: Muscle contraction at the molecular level.
Figure 1: Muscle contraction at the molecular level.
An international team led by a RIKEN researcher has begun to unravel some of the complex molecular details as to how muscles function.
The area is a hot topic of research because understanding how muscles work could lead to better treatment for medical conditions such as muscular dystrophy and some forms of heart disease. It could also provide a basis for developing more efficient artificial limbs and prosthetic devices and even for the production of biomotors for robots and microscopic devices.
At a molecular level, muscle contraction is a complicated interplay between two proteins, myosin and actin. Myosin molecules have a bulbous head (the motor domain) (Fig. 1) tacked onto a rod-like tail (the lever arm). Actin molecules join together in long chains to form a double-stranded helical filament. By alternately attaching to and detaching from the actin filament in a ratchet-like process, a myosin head ‘walks’ up its length, in the process pulling back on the filament and contracting the muscle. The energy for this process comes from splitting a phosphate group from he body’s standard energy molecule adenosine triphosphate (ATP).
The research group, based at RIKEN’s SPring-8 Center in Harima, investigated he details of the molecular attachment process to the actin filament1. From earlier work using crystallography and electron microscopes, the researchers determined an area or ‘patch’ on the head of the myosin molecule where the actin filament was bound. This patch is surrounded on three sides by molecular loops sticking out from the myosin head. These loops appeared to play a key role in binding the myosin head to the actin filament.
The researchers made changes to the structure of each of these loops, and then assessed the impact of their interference on the binding process. The loops surrounding the patch are guided into alignment with the actin filament by means of a weak interaction between the myosin head and the filament.
At this point, each of the loops becomes involved in specific strong binding with the actin filament. The result is to change the shape of the myosin head, closing a cleft in one place, while in another uncovering the bound ATP molecule and providing a pathway for its terminal phosphate group to split off. Thus, as the proteins bind together, the myosin is prepared to cleave its ATP molecule which provides it the energy to detach and thrust its head further up the length of the actin filaments.
“This study is just the beginning of the understanding of actin activation,” says Hirofumi Onishi of the SPring-8 Center. “The binding to actin triggers the whole of the later movement of the head. I want to continue this line of work using the same strategy.”
References
- 1. Onishi, H., Mikhailenko, S.V. & Morales, M.F. Toward understanding actin activation of myosin ATPase: the role of myosin surface loops. Proceedings of the National Academy of Sciences USA 103, 6136–6141 (2006). doi: 10.1073/pnas.0601595103
