Jan. 5, 2007 Research Highlight Biology
How a protein regulates cell structure
Japanese researchers unveil details of a key molecular interaction
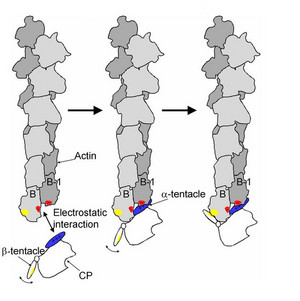 Figure 1: A model of the interaction of the capping protein (CP) with the barbed-end of an actin filament as proposed by the research team. EMBO/European Molecular Biology Organization/25/5632 (2006)
Figure 1: A model of the interaction of the capping protein (CP) with the barbed-end of an actin filament as proposed by the research team. EMBO/European Molecular Biology Organization/25/5632 (2006)
A RIKEN research team has combined electron microscopy with sophisticated image analysis to reveal details of a molecular interaction that regulates the activity of actin filaments, a key element in cellular structure and function.
Actin filaments are double-stranded helical chains or polymers of which the protomer, or basic unit, is the globular protein actin. These filaments form a contractile cellular skeleton which plays an important role in cell division, enables cells to move and assists the migration of protein complexes and organelles within cells. Controlling the activity of the filaments themselves is fundamental to cellular function.
Much of the activity of the filaments depends on their ability to lengthen and shorten by gaining or losing actin protomers. They do this preferentially at the end known as the barbed-end, as opposed to the pointed-end. This capability is inhibited by means of a capping protein that binds to the barbed-end, and locks in place. In a recent paper in EMBO1, the research team proposes a convincing model of how this molecular interaction occurs.
The team took high resolution electron micrographs of the barbed-ends of filaments with the capping proteins literally frozen in place. Subsequent image analysis using a novel procedure developed by the team revealed the orientation of the capping protein when bound to the filament.
The structure of the capping protein has been published previously—a globular body from which two tentacles, labeled α and β, protrude. In the orientation shown in the team’s electron micrographs, the α-tentacle lies across the two exposed actin protomers at the barbed-end of the filament. A region of basic, positively charged amino acids on the tentacle, sits next to negatively charged areas of acidic amino acids on each of the actin protomers.
The research team proposes that the electrostatic attraction between these areas draws the capping protein into position (Fig. 1). Once there, the freely swinging β-tentacle locks the capping protein into place as it is repelled by water into a position along the outer side of one of the actin protomers. This model fits with previous observations that while deletion of the α-tentacle severely weakens the interaction between capping protein and filament, deletion of the β-tentacle has a much milder effect.
The research team is now working on elucidating the structure of the pointed-end, says research team member Akihiro Narita of the RIKEN SPring-8 Center in Harima. “The difference between the two ends is one of the basic features of actin filaments.”
References
- 1. Narita, A., Takeda, S., Yamashita, A. & Maéda, Y. Structural basis of actin filament capping at the barbed-end: a cryo-electron microscopy study. The EMBO Journal 25, 5626–5633 (2006) doi: 10.1038/sj.emboj.7601395
