Feb. 16, 2007 Research Highlight Chemistry
Selection with silicon
Organic chemists develop a new one-pot reaction to make compounds containing carbon-silicon bonds

A new, simple synthetic method to selectively prepare compounds containing carbon-silicon bonds has been developed. These compounds—known as organosilyl compounds—exhibit unique structural, electrical, optical and chemical properties. It is these properties that make these compounds so attractive to researchers and they are used in various ways in material sciences, biotechnology, and organic synthesis.
The challenge for organic chemists is the selective synthesis of specific organosilyl compounds which also contain other reactive groups. To date, only a few practical methods have been developed. Now, Masanobu Uchiyama from the RIKEN Discovery Research Institute, Wako, and colleague Shinji Nakamura of The University of Tokyo, have developed a selective method they believe will be of use to many organic chemists1.
This new method treats alkenes, simple carbon-carbon double bonds, with complex catalysts containing silicon-zinc bonds to give the organosilyl compound. In the reaction, the silicon atom adds preferentially to the very end of the double bond and where there are two carbon-carbon double bonds in a molecule only the terminal one reacts leaving the other intact. This means that a range of starting compounds can be used. The method can also be modified to give compounds that have other functional groups adjacent to the silicon atom .
Practically speaking the reaction is also attractive as this is the first example of a ‘one-pot’ method to generate such compounds. The reaction takes place smoothly using an equivalent amount of reagent to starting compound. Uchiyama hopes this reaction could be applied to starting materials that, for example, once reacted, would form specifically just one of two possible mirror-image products, known as enantiomers.
“One of our aims is to provide novel approaches to functionalizing organic compounds by means of development of the chemistry of newly designed complexes. Success here will provide powerful tools for designing and creating new functionalized molecules,” says Uchiyama.
Uchiyama and Nakamura began research in this area in 2004—designing complexes and catalysts containing silicon-zinc bonds to synthesize functionalized carbon-carbon double and triple bonds2, 3. Tools for the selective introduction of various functional groups to organic molecules are still quite limited in their applicability. A fact that motivates the work undertaken in Uchiyama’s laboratory where they focus on the development of breakthrough synthetic processes to create new materials based on synthetic organic chemistry, physical chemistry and computational chemistry. The next step is to understand in detail how the reaction works and determine its scope.
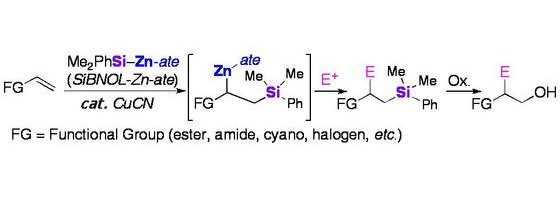 Regio- and chemoselective silylation of functionalized terminal alkenes.
Regio- and chemoselective silylation of functionalized terminal alkenes.
References
- 1. Nakamura, S. & Uchiyama, M. Regio- and chemoselective silylmetalation of functionalized terminal alkenes. Journal of the American Chemical Society 129, 28–29 (2007). doi: 10.1021/ja066864n
- 2. Nakamura, S., Uchiyama, M. & Ohwada, T. Chemoselective silylzincation of functionalized terminal alkynes using dianion-type zincate (SiBNOL-Zn-ate): regiocontrolled synthesis of vinylsilanes. Journal of the American Chemical Society 126, 11146–11147 (2004). doi: 10.1021/ja047144o
- 3. Nakamura, S., Uchiyama, M. & Ohwada, T. Cp2TiCl2-catalyzed regio- and chemoselective one-step synthesis of gamma-substituted allylsilanes from terminal alkenes using dianion-type aincate (SiSiNOL-Zn-ate). Journal of the American Chemical Society 127, 13116–13117 (2005). doi: 10.1016/j.neures.2017.07.008
