Mar. 9, 2007 Research Highlight Chemistry
Calculated approach to catalysis
Computer model helps uncover the mysteries behind the selective introduction of metal atoms to small organic molecules
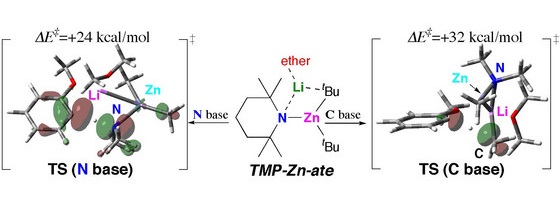 Figure 1: Visualization of reaction pathways and mechanism of TMP-Zincate has been studied using a computational model
Figure 1: Visualization of reaction pathways and mechanism of TMP-Zincate has been studied using a computational model
A theoretical study by chemists in Japan and the US has revealed the origin of differences in selectivity of a chemical reaction between two types of bimetallic reagents used to introduce metal atoms to aromatic rings. For decades, the choice of catalyst for a successful organometallic reaction has depended on which reactive group components were on the starting material. Moreover, a complete understanding of some of the difficulties encountered and of the underlying reaction mechanisms was lacking in many cases.
Now, Masanobu Uchiyama from the RIKEN Discovery Research Institute, Wako, and colleagues, which includes collaboration with Keiji Morokuma from Emory University, US, and Kyoto University, use a computational model that provides a rationale for the differences in the mechanisms between the two bimetallic reagents studied1. The first reaction involved an intermediate in which the two metal atoms were the same, as for traditional alkyllithiums or Grignard reagents; and the second involved an intermediate containing two different metal atoms, in this case a lithium-zincate complex—known as an ‘ate complex’.
These differences have implications for chemists wishing to develop better methods for improved reactivity and selectivity. The teams’ work also gives an insight into why reactions sometimes result in a product containing two metal atoms as opposed to one.
Their research emphasizes the importance of applying established theoretical methods to rational catalyst design (Fig. 1). Uchiyama understands that traditional approaches to design new reactions based on past experiences, or trial and error, are often expensive and time-consuming. He believes a dual approach combining the benefits of theoretical and experimental chemistry should be encouraged: an approach that will result in breakthrough synthetic processes, use fewer chemicals and be more cost effective.
This work is the result of a collaboration that started in 2001 between Uchiyama and several colleagues from The University of Tokyo and RIKEN. The collaboration has already resulted in five other publications. Uchiyama acknowledges that his colleagues play a central role in this research. “We have been working hard the past several years to clarify the unknown reaction pathways and mechanisms of small molecules and macromolecules,” he says.
The next steps in Uchiyama’s work, the ‘flexible and command’ construction of aromatic compounds with specific functional group arrangements, will be of interest to medicinal and materials chemists. “We intend to develop new transformations on aromatic rings that have unique reactivity and selectivity using these results in combination with our experiences as organometallic chemists,” says Uchiyama.
References
- 1. Uchiyama, M., Matsumoto, Y., Usui, S., Hashimoto, Y. & Morokuma, K. Origin of chemoselectivity of TMP zincate bases and differences between TMP zincate and alkyl lithium reagents: A DFT study on model systems. Angewandte Chemie International Edition 46, 926–929 (2007). doi: 10.1002/anie.200602664
