Apr. 6, 2007 Research Highlight Biology
Neurons transmit signals in reverse
Information ‘feedback’ drives communication between neurons
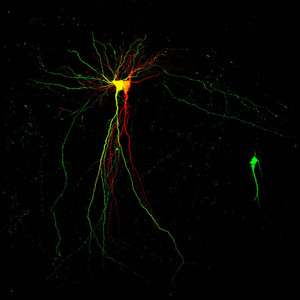 Figure 1: Two pyramidal neurons forming a synapse: post-synaptic green-yellow cell (left) and pre-synaptic red cell (right).
Figure 1: Two pyramidal neurons forming a synapse: post-synaptic green-yellow cell (left) and pre-synaptic red cell (right).
Researchers at the RIKEN Brain Science Institute, Wako, and the RIKEN-MIT Neuroscience Research Center, Cambridge, US, find that some signals transmitted between successive neurons move forward while other signals move in reverse. Such ‘backward’ signal transmission provides essential information to earlier neurons that serves to coordinate brain activity.
A collaboration of scientists led by Kensuke Futai and Yasunori Hayashi measured chemical-electrical signal propagation neurons. They found that backward, or ‘retrograde’, signal transmission between neurons is required for coordinated neuronal activity1. Those results and work by others challenge the long-held belief that synaptic transmission moves in only one direction.
Neurons in the brain must be coordinated for thoughts and actions to occur. The challenge of coordinating neuronal activity, which can rightly be viewed as a ‘problem of coordination’, stems from the trillions of individual neurons that communicate through synapses (Fig. 1), the diversity of neuronal connections in the brain, and the continuing development of new connections when memories are formed. How, then, do neurons coordinate their activities?
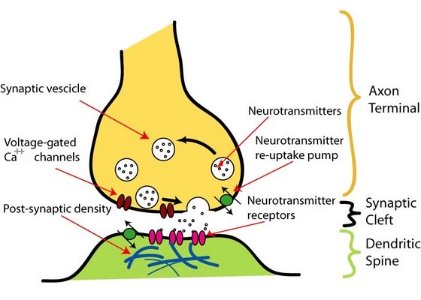 Figure 2: Schematic diagram of an axonal pre-synaptic terminal and a dendritic post-synaptic terminal. Source: Wikipedia
Figure 2: Schematic diagram of an axonal pre-synaptic terminal and a dendritic post-synaptic terminal. Source: Wikipedia
Chemical-electrical signals that arise in neuron cell bodies propagate forward along neuron axons and then traverse synaptic clefts (or spaces) between pre- and neighboring post-synaptic neuron ‘terminals’ (Fig. 2). Because pre- and post-synaptic terminal size and function are intimately linked, Futai and colleagues speculated that signals from post-synaptic terminals directly influence the function and properties of pre-synaptic terminals.
To test that hypothesis and to determine a mechanism for possible retrograde signal transmission, the team evaluated two post-synaptic proteins: PSD-95 and neuroligin, which associate with each other. Neuroligin also extends across synaptic clefts to interact with a pre-synaptic protein called β-neurexin. The physical connection between post- and pre-synaptic termini mediated by PSD-95, neuroligin and β-neurexin regulates the release of chemical neurotransmitter from pre-synaptic termini.
By measuring the chemical-electrical communication between neurons, the team found that the amount of PSD-95 and neuroligin protein in post-synaptic termini is directly proportional to the probability that neurotransmitter would be released from pre-synaptic termini. This result demonstrates that ‘reverse’ signaling directly affects pre-synaptic function.
Implications of this work by the RIKEN team may include insight into diseases such as autism and Alzheimer’s. As Futai remarks, “our study might have put the spotlight [on] the molecular mechanism of autism”, alluding to a recent study in Nature Genetics that links mutations in neuroligin and an increased incidence of autism. Perhaps more intriguingly, the team’s work may also provide insight into memory formation.
References
- 1. 1. Futai, K., Kim, M.J., Hashikawa, T., Scheiffele, P., Sheng, M. & Hayashi, Y. Retrograde modulation of presynaptic release probability through signaling mediated by PSD-95–neuroligin. Nature Neuroscience 10, 186–195 (2007). doi: 10.1038/nn1837
