Jun. 29, 2007 Research Highlight Biology
Artificial lymph nodes induce strong immune response
Researchers open the way to new therapy for infection and cancer
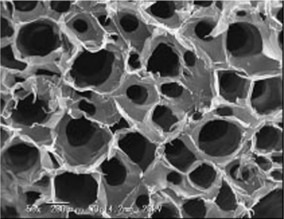 Figure 1: Micrograph of a section of the collagen scaffold used to construct the artificial lymph nodes.
Figure 1: Micrograph of a section of the collagen scaffold used to construct the artificial lymph nodes.
A RIKEN research team, which developed functioning artificial lymph nodes (aLNs), has now shown their nodes can generate immune cells and strong immunological responses when transplanted into mice lacking a working immune system1.
The research is a significant step towards being able to strengthen or perhaps even replace human immune systems. It has particular relevance to the fight against AIDS, cancer, and intractable infectious diseases, and also to treating allergies. “We want to make a prototype human model within two or three years,” says project leader Takeshi Watanabe.
The researchers, from RIKEN’s Research Center for Allergy and Immunology in Yokohama, constructed their mouse aLNs by impregnating two- to three-millimeter-diameter scaffolds of the fibrous structural protein collagen with dendritic cells and stromal cells extracted from the thymuses of newborn mice (Fig. 1).
Earlier work suggested that it is the stromal cells that are responsible for organizing the structure of lymph nodes, which house immune system cells involved in screening the body for pathogens and toxins. The researchers tested this hypothesis by implanting aLN scaffolds into mice transgenic for green fluorescent protein, so the cells of the recipient mouse were easily distinguishable from those which had been introduced. They found that most of the immune cells in the developing aLNs derived from the recipient mouse, suggesting the role of the implanted tissue was not to generate immune cells, but to organize those already present into an operational unit.
Testing the artificial lymph nodes
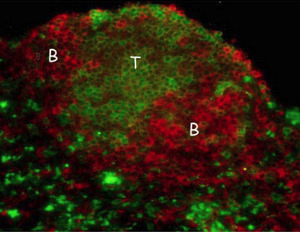 Figure 2: A section through the tissue of a developed artificial lymph node showing domains of T-cells and B-cells (labelled). © American Society for Clinical Investigation (2007)
Figure 2: A section through the tissue of a developed artificial lymph node showing domains of T-cells and B-cells (labelled). © American Society for Clinical Investigation (2007)
The researchers then checked how well their aLNs functioned. For this work, the aLNs were implanted initially into mice with a normal, healthy immune system, which had previously been injected with a harmless antigen compound to trigger an immune response. So the aLNs became populated with immune system T-cells and B-cells which specifically recognized and countered microbes or cancer cells expressing the injected antigen (Fig. 2).
These primed aLNs were then transplanted into two sets of mice—a group with a normal immune system which had never been exposed to the antigen, and a group in which the immune system did not function. When later exposed to the antigen both groups responded immediately by making the appropriate protective antibodies—and the response to the antigen lasted for longer than four weeks, which suggests that specific immune cells had been generated which retained the ‘memory‘ of the antigen (Fig. 3).
Tracking the migration of immune cells
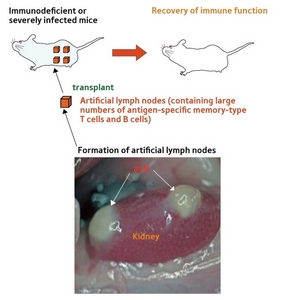 Figure 3: Artificial lymph nodes could be used in therapy to target specific disease conditions.
Figure 3: Artificial lymph nodes could be used in therapy to target specific disease conditions.
Further investigation using aLN tissue tagged with green fluorescent protein showed that in immunodeficient mice, T- and B-cells from the aLNs migrated to repopulate the spleens and bone marrows which lacked such immune cells. Once in these organs, the immune cells generated large numbers of antigen-specific antibody-forming cells. When tagged aLNs were transplanted into mice with normally functioning immune systems, however, the antibody-forming cells did not migrate, but remained in the aLNs. So the migration of immune cells to take up residence in the spleen and bone marrow only occurred in mice which were immune-cell free.
The researchers then studied the migration process further. Previous work had suggested that the movements of migrating immune cells are directed by the signals generated by groups of receptors associated with guanine-nucleotide-binding or G proteins. The research team was able to test this idea using a bacterial toxin which blocks the action of G proteins. Culturing aLNs in this toxin before implantation dramatically decreased the numbers of immune cells which migrated to the spleen and bone marrow in immunodeficient mice.
The team undertook a similar experiment using a compound which blocks one specific type of G-protein-associated receptor based around sphingosine-1-phosphate. Again this action inhibited migration of immune cells from the implanted aLNs to the spleen and bone marrow of immunodeficient mice.
The successful development of the aLNs in mice opens the way to producing customized lymph nodes impregnated with antibody-forming cells and other compounds specifically geared to treating certain conditions. “That is our purpose,” says Watanabe, “not necessarily to make replacements for natural lymph nodes, but rather more functional organs applicable to particular diseases and allergies.”
References
- 1. Okamoto, N., Chihara, R., Shimizu, C., Nishimoto, S. & Watanabe, T. Artificial lymph nodes induce potent secondary immune responses in naïve and immunodeficient mice. The Journal of Clinical Investigation 117, 997–1007 (2007). doi: 10.1172/JCI30379
About the Researcher
Takeshi Watanabe

Takeshi Watanabe was born in Osaka, Japan, in 1940. He graduated from Osaka University’s School of Medicine in 1965 before obtaining his MD and PhD degrees from the same university in 1966. He then worked as a physician at the university until 1969, before he left to undertake post-doctoral training at Roswell Cancer Institute in Buffalo, US. In 1972, Watanabe returned to the Osaka University School of Medicine and served as an assistant professor until 1980. During that time, he also worked as a researcher at Base Institute for Immunology in Basel, Switzerland for two years. In 1980, he attained his full professorship at Saga Medical School, Japan. From 1985 to 2004, he worked as a professor at the Medical Institute of Bioregulation, Kyushu University in Fukuoka, Japan, where he concurrently served as the director from 2001. Watanabe is currently a unit leader at the RIKEN Research Center for Allergy and Immunology.
