Nov. 17, 2008 Research Highlight Biology
Pass the protein
Transport of the Otx2 protein from the eye to the brain may initiate visual cortex plasticity
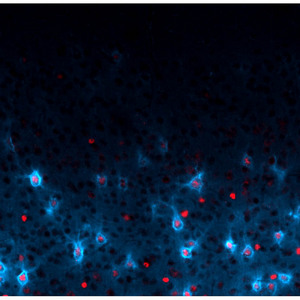 Figure 1: The protein Otx2 (red) controls the maturation of PV-cells (blue). These cells are thought to be involved in visual cortex plasticity.
Figure 1: The protein Otx2 (red) controls the maturation of PV-cells (blue). These cells are thought to be involved in visual cortex plasticity.
Visual information from both eyes finally comes together when it is transmitted to the primary visual cortex. If one of the eyes is deprived of visual input during a ‘critical period’ of postnatal development, the representation of the non-deprived eye within the visual cortex expands at the expense of the deprived eye. This leads to a permanent loss of visual acuity (amblyopia) in the deprived eye.
The timing of this visual experience-dependent cortical plasticity requires a particular population of inhibitory interneurons within the visual cortex that express the marker parvalbumin (PV). Now, an international team of scientists, led by Takao Hensch at the RIKEN Brain Science Institute in Wako, has found that a protein called Otx2 is responsible for the final maturation of these cells (Fig. 1), and is therefore required for establishing the time during which visual cortex plasticity occurs1.
The comings and goings of Otx2
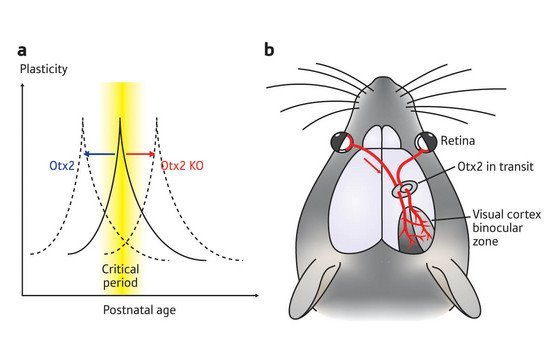 Figure 2: (a) The shift in critical period for visual cortex plasticity with the infusion of Otx2 protein (blue) and in mice lacking the protein (red). (b) Otx2 protein is transported from the eye of immature mice to the visual cortex to induce maturation of PV interneurons.
Figure 2: (a) The shift in critical period for visual cortex plasticity with the infusion of Otx2 protein (blue) and in mice lacking the protein (red). (b) Otx2 protein is transported from the eye of immature mice to the visual cortex to induce maturation of PV interneurons.
Otx2 is a transcription factor, a type of protein that is involved in determining cell fate. Otx2 is expressed during embryonic development, and is necessary for formation of the head. Other groups had reported that Otx2 disappears from the rodent brain by postnatal day 13, but its presence at later points in development had not been examined. Surprisingly, Hensch and his colleagues found Otx2 protein re-emerging in the mouse brain by postnatal day 29. This re-appearance of Otx2 occurred within the sparse set of PV interneurons in parallel with the critical period of visual cortex plasticity.
The presence of Otx2 protein in the cortex required visual experience: when the researchers deprived mice of visual input by, for example, keeping them in the dark from birth, they observed a decrease in Otx2 and PV expression in the visual cortex. PV cell maturation is therefore dependent on visual experience during postnatal development.
Direct delivery of Otx2 protein into the visual cortex restored PV expression in visually deprived mice. Mature PV cells are encircled by extracellular matrix molecules that make up a peri-neuronal net (PNN) around the cell, and these PNNs do not form normally in mice that are kept in the dark. Delivery of Otx2 protein into the cortex was also sufficient to induce PNN formation around PV interneurons in visually deprived animals.
Mice normally open their eyes for the first time at postnatal day 13, but the critical period for visual cortex plasticity does not begin until at least a week later. The researchers found that infusion of Otx2 protein into the visual cortex of immature mice, at postnatal day 17, was able to shift the timing of the critical period for visual cortex plasticity to this earlier point in postnatal development (Fig. 2a). In addition, Otx2 infusion accelerated the maturation of the PV interneurons in the visual cortex.
Hensch and his colleagues then examined the visual cortex of mice that lacked Otx2. These Otx2-deficient mice had few PV-expressing cells and exhibited abnormal PNN development in their visual cortex, and did not show evidence of visual cortex plasticity (Fig. 2a).
Interestingly, even though Otx2 was required for the formation of the PV inhibitory interneurons, Otx2 was not produced within the cells that needed it. Instead, Otx2 was synthesized in distant structures involved in vision, such as the eye, and seemed to be transported to the cortex during visual activity (Fig. 2b): when the researchers injected a labeled Otx2 protein into the eye, they found that it showed up in the visual cortex within PV-expressing cells.
Hensch says that he and his colleagues “would like to visualize the transport process and to understand why these proteins are specifically attracted to PV cells in vivo. Both points will illuminate novel cell biology about how these classical molecules work.”
Shaping plasticity
The researchers’ findings indicate that the Otx2 made in the eye could be transported along the pathway that carries visual information to the visual cortex. The Otx2 protein would then be taken up by immature cortical PV cells so that they can become fully functional interneurons.
“Surprisingly, the onset of brain plasticity may not be determined by an intrinsic cortical program but rather by a molecular messenger delivered from below,” says Hensch. “In other words, the eye is telling the brain when to rewire. It would be exciting to know if other sensory modalities are similarly shaped by a uniquely transported protein.”
The researchers further demonstrated that Otx2 production in the eye, not the cortex, might underlie visual cortex plasticity. First, they showed that inhibition of Otx2 expression in the eye could inhibit plasticity in the visual cortex. On the other hand, when they attempted to block Otx2 production in the cortex, they did not observe any effect on visual cortex plasticity. Finally, infusing an antibody that traps Otx2 as it passes from the eye to the cortex was sufficient to inhibit visual cortex plasticity. Their results suggest that Otx2 is created in the eye, but acts in the visual cortex to induce plasticity.
An eye to the future
These findings raise the general possibility that cell-to-cell transmission of similar proteins may also be responsible for plasticity in other areas of the nervous system. This may have implications for understanding brain processes that are associated with plasticity, such as drug addiction.
“Beyond amblyopia, many psychiatric or cognitive developmental disorders, such as autism or schizophrenia, may be thought of as critical period disorders,” says Hensch. “If we can now access and affect cortical plasticity from the periphery, one might imagine exciting new routes for therapy or recovery from brain injury in adulthood.”
References
- 1. Sugiyama, S., Di Nardo, A.A., Aizawa, S., Matsuo, I., Volovitch, M., Prochiantz, A. & Hensch, T.K. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell 134, 508–520 (2008). doi: 10.1016/j.cell.2008.05.054
About the Researcher
Takao K Hensch

Takao K Hensch was born in Tokyo, Japan, in 1966. He graduated from Harvard University in 1988, the University of Tokyo (MPH, 1991) and was a Fulbright Fellow at the Max-Planck Institut fuer Hirnforschung prior to obtaining his PhD in Neuroscience from the University of California, San Francisco (1996). He then returned to Japan as a Laboratory Head to help launch the new Brain Science Institute at RIKEN, and was promoted to Group Director of Critical Period Mechanisms Research in 2000. He is now joint Professor of Neurology (Children’s Hospital Boston) and Molecular & Cellular Biology at Harvard University. His research focuses on the structural and functional rewiring of cortical connections in response to early sensory experience with emphasis on cognitive developmental disorders. He has received the Tsukahara Prize (2001), Minister of Science Award (2006) and the first US Society for Neuroscience Young Investigator Award to a foreign scientist (2005).
