Nov. 9, 2012 Research Highlight Biology
Helping the nervous system pull itself together
A cascade of signaling proteins triggers a cellular ‘tug of war’ that creates the foundation for the embryonic central nervous system
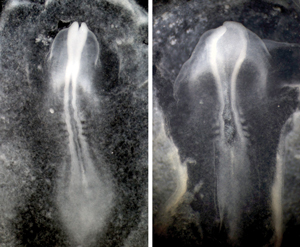 Figure 1: In a normal chick embryo (left), the protein Celsr1 helps to induce proper formation of the neural tube. In chick embryos lacking this protein (right), the neural plate fails to curve so cannot close properly. © 2012 Masatoshi Takeichi, RIKEN Center for Developmental Biology
Figure 1: In a normal chick embryo (left), the protein Celsr1 helps to induce proper formation of the neural tube. In chick embryos lacking this protein (right), the neural plate fails to curve so cannot close properly. © 2012 Masatoshi Takeichi, RIKEN Center for Developmental Biology
In developing vertebrates, the brain and spinal cord originate from an embryonic structure known as the neural tube. This initially forms as a flat ‘neural plate’, which subsequently folds around and closes up to form a tubular structure. By performing an extensive series of experiments in developing chick embryos, Masatoshi Takeichi and his team at the RIKEN Center for Developmental Biology, Kobe, has now revealed valuable insights into the mechanism underlying the closure process1.
The neural plate is formed from epithelial cells, which feature clearly defined ‘top’ (apical) and ‘bottom’ (basal) surfaces. At the apical surface, the cells are connected by structures called adherens junctions, which are in turn connected to a network of actomyosin protein fibers. “It is known that the contraction of these actomyosin fibers causes bending of these epithelial sheets in the apical direction through the constriction of their apical surfaces,” says Takeichi. “We became interested in learning how this mechanism contributes to the formation of the neural tube.”
The initial bending of the neural plate is most prominent near the midpoint of what will ultimately become the brain and spinal cord. Within this embryonic region, Takeichi and colleagues observed that neural plate cells tended to form polarized ‘chains’ of actomyosin fibers that extend across multiple cells, perpendicular to the head–tail axis of the embryo. As closure begins, the cells undergo extensive rearrangement in a process called ‘convergent extension’. Cells of the neural plate squeeze inward, pushing adjacent cells forward and backward and resulting in lengthwise extension of the developing neural tube.
The researchers determined that a protein called Celsr1 helps direct this convergent extension (Fig. 1). They subsequently identified a network of proteins that act downstream of Celsr1. Together, these proteins form a signaling cascade that induces the contraction of the long actomyosin fibers that tether the neural plate epithelial cells together. As the fibers contract, these cells’ apical surfaces become pinched toward the midline of the neural plate, and this steady narrowing of the apical surface ultimately results in the curvature of plate.
These findings explain a critical step in the development of the nervous system, and could help illuminate the roots of conditions arising from faulty neural tube development, like spina bifida. “Mice in which these genes are mutated show neural tube closure defects,” says Takeichi, “and irregularities in any of the genes or molecules identified here could cause birth defects [in humans].”
References
- 1. Nishimura, T., Honda, H. & Takeichi, M. Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell 149, 1084–1097 (2012). doi: 10.1016/j.cell.2012.04.021
