May 17, 2013 Research Highlight Chemistry
Gold atoms caught in the act
Femtosecond ‘snapshots’ reveal a dramatic bond tightening in photo-excited gold complexes
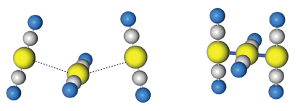 Figure 1: A gold(I)–dicyanide trimer chain (left) changes from a bent geometry to a linear structure with short gold–gold bonds (right) on photoexcitation. Reproduced, with permission, from Ref. 1 © 2013 American Chemical Society
Figure 1: A gold(I)–dicyanide trimer chain (left) changes from a bent geometry to a linear structure with short gold–gold bonds (right) on photoexcitation. Reproduced, with permission, from Ref. 1 © 2013 American Chemical Society
Metal complexes are becoming increasingly important as the photochemical building blocks of functional molecular systems such as sensors and photoelectrochemical cells. Of particular interest are metal complexes that involve gold atoms in the +1 valence state, due to their ability to self-assemble into larger units. The assembly process, known as aurophilic interaction, is enhanced by photoexcitation—an effect recently exploited by chemists to link individual gold(I)–dicyanide complexes into phosphorescent oligomer chains through careful control of complex concentrations and light exposure. However, the fundamental structural details of this reaction have yet to be understood.
Tahei Tahara and colleagues from the Molecular Spectroscopy Laboratory at RIKEN, in collaboration with scientists from Toyama University, have now used ultra-fast laser spectroscopy to observe the movements of gold atoms in real time during the photochemical reaction of gold(I)–dicyanide1.
To make their observations, Tahara and his colleagues used a laser spectroscopy technique that is capable of resolving changes in molecular emission and absorption at the femtosecond scale—a method that the researchers had previously used to observe the flattening of a propeller-like copper complex. This time, the team studied how ‘trimers’ of three linked gold(I)–dicyanide molecules are affected by light. Theory predicts a rapid decrease in distances among gold atoms when electrons are pushed into a photoexcited state. However, no experimental evidence exists for this sort of bond tightening.
In response to laser excitation, the gold(I)–dicyanide trimer produced a new absorption signal that progressively grew more intense and shifted to higher energies over a period of 2 picoseconds, which the team ascribed to an excited-state trimer undergoing a geometric change. Density functional theory (DFT) calculations revealed this timeframe to be consistent with a bent-to-linear transformation of the trimer and tight gold–gold bond formation.
By scrutinizing the first picosecond of spectral data given off by the excited-state trimer, the researchers saw that the peak absorption signal oscillated sharply in femtosecond increments. Tahara notes that this kind of modulation is clear evidence of a rapid shortening of inter-atomic gold distances (Fig. 1)—an interpretation backed by DFT computations correlating the oscillation frequencies to gold–gold stretching vibrations. The team’s results also show that the bent-to-linear transformation must occur before the gold(I) complexes can link into longer oligomers. The researchers anticipate that the fundamental insights gained using ultra-fast laser spectroscopy will prove critical to chemists’ understanding of the light-induced self-assembly of metal complexes.
References
- 1. Iwamura, M., Nozaki, K., Takeuchi, S. & Tahara, T. Real-time observation of tight Au–Au bond formation and relevant coherent motion upon photoexcitation of [Au(CN)2–] oligomers. Journal of the American Chemical Society 135, 538–541 (2013). doi: 10.1021/ja310004z
