Jun. 28, 2013 Research Highlight Medicine / Disease
Experimental compound offers fragile X treatment
Studies in mice indicate that a daily dose of a kinase inhibitor could reverse the underlying morphological defects responsible for fragile X syndrome
 Figure 1: Mice with fragile X syndrome (FXS) but expressing neither the PAK nor FMRP proteins display greatly improved symptoms. © iStockphoto/ Thinkstock
Figure 1: Mice with fragile X syndrome (FXS) but expressing neither the PAK nor FMRP proteins display greatly improved symptoms. © iStockphoto/ Thinkstock
Fragile X syndrome (FXS) is the most common inherited form of autism and intellectual disability, characterized by hyperactivity, repetitive behaviors and seizures. Although there are a number of medications that can help individuals to cope with the behavioral and mental health issues associated with the syndrome, as yet there are no proven therapies that directly impact the root cause of the disease. However, this could now be about to change with the RIKEN-led discovery of a compound that not only treats the symptoms in adult mice but also reverses the morphological defects associated with the syndrome1.
The international research team, led by Susumu Tonegawa of the RIKEN–MIT Center for Neural Circuit Genetics (CNCG) at the Massachusetts Institute of Technology (MIT) in the United States, recently demonstrated the remarkable effectiveness of their promising compound—called FRAX486—in model FXS mice. “The effect was dramatic,” says Tonegawa, a Nobel laureate and also director of the RIKEN Brain Science Institute. “With only one dose, the mouse was cured.”
The development of FRAX486 began six years ago when Tonegawa and his colleagues were studying mice engineered to have low activity of an enzyme known as p21-activated kinase (PAK). Unexpectedly, they noticed that the animals had relatively few dendritic spines in their brains, and the spines that were there tended to be short and stubby. Dendritic spines are small neuronal protrusions that assist with neuron-to-neuron communication in the synapse. The researchers recognized that the absence of these spines in PAK-mutant mice was the exact opposite of what is seen in people with FXS and in mouse models of the disease. In FXS mice, which lack a working copy of the gene that encodes the fragile X mental retardation protein (FMRP), researchers typically see a profusion of long and thin dendritic spines in the brain.
The observation that mice lacking PAK and FMRP display mirror-opposite phenotypes led Tonegawa’s team to test an intriguing hypothesis: perhaps PAK and FMRP antagonize each other to regulate spine morphology. Inhibiting PAK activity might therefore correct some of the defects associated with FXS. To test this idea, the researchers engineered a mouse to express neither PAK nor FMRP. To their surprise and delight, these mice had normal numbers of dendritic spines, unlike those only missing FMRP. Mice lacking both proteins were also less anxious and displayed fewer repetitive behaviors than FXS model animals. “It looked like the mouse was cured,” says Tonegawa. “We never thought it would come out that nicely,” he adds.
A promising venture
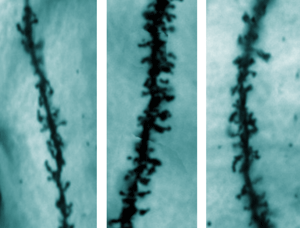 Figure 2: The brains of FXS mice treated with FRAX486 display a similar density of dendritic spines (right) to that of healthy mice (left), and a lower density than the brains of FXS mice given a sham treatment (center). Reproduced from Ref. 1 © 2013 B. M. Dolan et al.
Figure 2: The brains of FXS mice treated with FRAX486 display a similar density of dendritic spines (right) to that of healthy mice (left), and a lower density than the brains of FXS mice given a sham treatment (center). Reproduced from Ref. 1 © 2013 B. M. Dolan et al.
In 2007, soon after the team’s first promising results were published, Tonegawa was contacted by Jay Lichter of Avalon Ventures—a US venture capital firm. Lichter was interested in working with Tonegawa to develop a drug that could chemically inhibit PAK. They hoped that such an agent could yield the same therapeutic benefit as the genetic manipulation reported by Tonegawa’s research team but be applicable as a treatment in humans. Later that year, Tonegawa and Lichter founded the biotechnology company Afraxis to further develop the PAK inhibitor concept.
Scientists at Afraxis embarked on a search for a potent inhibitor of PAK by screening a library of 12,000 small molecules known to block kinase enzymes. Their search yielded one promising hit: FRAX486, a compound that selectively inhibits its target and also readily crosses the blood–brain barrier, staying active in the brains of mice for many hours. The researchers found that a daily dose of FRAX486 achieved a steady state of active drug levels in the brain.
Next, Tonegawa’s research group undertook further characterization of the FRAX486 compound. This required new tests to be developed to quantify the effectiveness of the drug. Correspondingly, Bridget Dolan, a member of Tonegawa’s laboratory at the CNCG, created an assay that involves the measurement of sound-induced seizures in FXS mice. She found that most mice injected with higher doses of FRAX486 were immune to seizures 8 hours after treatment, whereas all untreated FXS mice suffered seizures, with around half subsequently dying from respiratory arrest.
Face the FRAX
Dolan also showed that treating FXS mice with the drug ameliorated the autism-like symptoms of the disorder, such as hyperactivity and circling behavior. Meanwhile, collaborators at the National Institute of Mental Health and Neuro Sciences in India demonstrated that FRAX486 rescued morphological abnormalities in FXS mice. Just 8 hours after a single dose of the drug, the density of dendritic spines in FXS mice returned to levels similar to those found in healthy mice and well below the density in sham-treated FXS animals (Fig. 2). Physiological tests performed by a research team at Seoul National University in South Korea also revealed that FRAX486 treatment had no impact on body weight.
Importantly, all the beneficial effects were seen in adult mice that had already developed many of the symptoms of FXS. “FRAX486 is not only preventative, but also curative after the mouse gets sick,” notes Tonegawa.
In early 2013, Genentech—a US-based subsidiary of the Swiss drug company Roche—acquired the patent portfolio behind FRAX486 and is now running further tests on the drug and related compounds with the hope of advancing a lead candidate into human trials in the near future.
References
- 1. Dolan, B. M., Duron, S. G., Campbell, D. A., Vollrath, B., Shankaranarayana Rao, B. S., Ko, H.-Y., Lin, G. G., Govindarajan, A., Choi, S.-Y. & Tonegawa S. Rescue of fragile X syndrome phenotypes in Fmr1 KO mice by the small-molecule PAK inhibitor FRAX486. Proceedings of the National Academy of Sciences USA 110, 5671–5676 (2013). doi: 10.1073/pnas.1219383110
About the Researcher
Susumu Tonegawa

Susumu Tonegawa received his PhD from the University of California, San Diego in the United States. He then undertook postdoctoral work at the neighboring Salk Institute for Biological Studies before moving to the Basel Institute for Immunology in Switzerland, where he performed his landmark immunology experiments. In 1987, Tonegawa was awarded the Nobel Prize in Physiology or Medicine for “his discovery of the genetic principle for generation of antibody diversity.” Using advanced genetic manipulation techniques, Tonegawa is now unraveling the molecular, cellular and neural circuit mechanisms that underlie learning and memory. He is currently Picower Professor of Biology and Neuroscience at the Massachusetts Institute of Technology (MIT) and director of both the RIKEN-MIT Center for Neural Circuit Genetics and the RIKEN Brain Science Institute.
