Aug. 23, 2013 Research Highlight Medicine / Disease
An eel protein illuminates human health
The unusual properties of a fluorescent protein produced in eel muscle could prove useful for both clinical diagnostics and specialized biomedical imaging applications
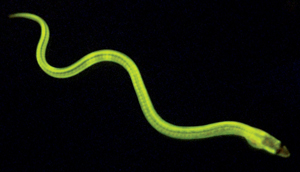 Figure 1: A green fluorescent protein produced in the Japanese freshwater eel is the first of its kind to be discovered in a vertebrate species. © 2013 Akiko Kumagai and Atsushi Miyawaki, RIKEN Brain Science Institute
Figure 1: A green fluorescent protein produced in the Japanese freshwater eel is the first of its kind to be discovered in a vertebrate species. © 2013 Akiko Kumagai and Atsushi Miyawaki, RIKEN Brain Science Institute
Visualizing individual molecules within the crowded cellular interior is a daunting task. Thanks to the discovery of naturally occurring fluorescent proteins in the 1990s, however, such experiments are now routine. Since the first successful cloning of the gene encoding green fluorescent protein (GFP) from a species of jellyfish, hundreds of other fluorescent proteins have been identified, giving biologists a remarkable array of tools with which to label and image specific molecules, cells or even entire tissues within living organisms.
Atsushi Miyawaki, head of the Laboratory for Cell Function Dynamics at the RIKEN Brain Science Institute in Wako, has spent much of his career searching for and studying fluorescent molecules. His team has now cloned an unusual fluorescent protein with particularly promising clinical utility1.
Most natural fluorescent proteins isolated to date have been purified from marine invertebrates such as jellyfish and coral. Until quite recently, no such proteins had been identified in vertebrate species. Miyawaki was therefore surprised to learn that Seiichi Hayashi, a food chemist at Kagoshima University, had isolated a GFP from the muscle of the Japanese freshwater eel, commonly known as unagi (Fig. 1).
After learning of Hayashi’s discovery, Miyawaki immediately purchased two eels at a local fish market. “I tried just shining light on the muscle, and I was astonished by the bright green fluorescence,” he says. “After that, I was very curious about the fluorescence in the eel muscle and set about cloning this gene.”
Providing long-range protection for muscles
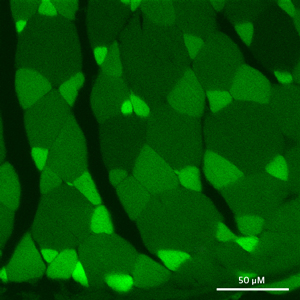 Figure 2: The UnaG fluorescent protein is produced primarily in a subset of thin muscle fibers, as seen in this cross-section. © 2013 Ryoko Ando and Atsushi Miyawaki, RIKEN Brain Science Institute
Figure 2: The UnaG fluorescent protein is produced primarily in a subset of thin muscle fibers, as seen in this cross-section. © 2013 Ryoko Ando and Atsushi Miyawaki, RIKEN Brain Science Institute
GFP and related proteins exhibit chemical properties that make them inherently fluorescent. This novel ‘UnaG’ protein, however, needs additional assistance from a chemical known as bilirubin in order to fluoresce. Bilirubin is an intermediate molecule in the degradation of heme—the iron-binding component of hemoglobin that gives red blood cells their distinctive color and enables them to transport oxygen throughout the body. UnaG recognizes and binds to bilirubin astonishingly fast, and the two molecules generate bright green fluorescence almost immediately after they combine.
Upon analyzing its sequence and structure, the researchers determined that UnaG belongs to the family of fatty-acid-binding proteins (FABPs), which typically store or transport lipid molecules. Compared to its relatives, however, UnaG is extremely choosy. “Members of the FABP family generally have low affinity for their ligand, and some are ‘broad-spectrum’ and bind to more than one fatty acid ligand,” says Miyawaki. “But UnaG only binds to bilirubin, and with very high specificity and high affinity.”
UnaG is produced in a subset of thin muscle fibers (Fig. 2), where it specifically recognizes what is known as ‘unconjugated’ bilirubin. This heme metabolite is subsequently transported by the blood protein albumin to the liver, where it is converted enzymatically to ‘conjugated’ bilirubin prior to its eventual excretion from the body. However, mounting evidence suggests that the body deliberately maintains a pool of unconjugated bilirubin, which acts as a potent antioxidant that protects tissues against damage from oxidative stress.
Unagi eels migrate for several months at a time, requiring extended muscle exertion that can generate the sort of chemicals that promote oxidative stress. Miyawaki hypothesizes that UnaG may offer a countermeasure to protect muscle tissue in such circumstances. “If you look at locusts or birds, which travel over long distances, their muscles show greatly increased expression of a type of FABP that probably works as a store for some kind of fatty acid for fuel,” he says. “In analogy, we think that the UnaG protein works as storage for this powerful antioxidant.”
A visible improvement in diagnostics
Several studies have suggested that antioxidant properties protect humans against disorders associated with oxidative stress, such as cardiovascular disease and metabolic syndrome. “A mild increase in bilirubin in our bodies is quite good,” says Miyawaki. On the other hand, excessive unconjugated bilirubin can be toxic, and infants in particular are vulnerable to bilirubin-induced brain damage—a potentially fatal condition known as kernicterus.
Doctors have been performing blood tests to detect elevated bilirubin for almost a century but continue to rely on the same slow and imprecise methodology of days past. “Technology for measuring bilirubin has shown very little evolution,” says Miyawaki, who previously trained as a physician. Since the speed with which UnaG generates a visible readout in the presence of unconjugated bilirubin could make it a superior alternative to these older methods, the researchers performed a head-to-head comparison. “With our kit, we were able to obtain reproducible results within 10 minutes,” says Miyawaki. UnaG exhibited at least a thousandfold greater bilirubin sensitivity than existing methods, and the researchers also found that the protein could be stored as a dried powder and subsequently reconstituted, making it suitable for integration into a ready-to-use diagnostic kit.
UnaG is in fact overly sensitive to bilirubin, and Miyawaki’s group is now using protein-engineering techniques to generate variants with finely tuned affinities that are better suited for use in clinical diagnostics and physiological research. These derivatives could also prove useful in research efforts to dissect the broader contributions of bilirubin to human health and disease. Among its other unique and potentially useful features, UnaG does not require oxygen to generate its glow, unlike other fluorescent proteins. This means UnaG could be used to illuminate anaerobic physiological and experimental environments where GFP would be snuffed out, such as the interior of a solid tumor—although Miyawaki points out that it remains to be seen whether bilirubin can effectively penetrate these dark corners. “We are going to find out the limitations of UnaG and how much it can contribute,” he says.
References
- 1. Kumagai, A., Ando, R., Miyatake, H., Greimel, P., Kobayashi, T., Hirabayashi, Y., Shimogori, T. & Miyawaki, A. A bilirubin-inducible fluorescent protein from eel muscle. Cell 153, 1602–1611 (2013). doi: 10.1016/j.cell.2013.05.038
About the Researcher
Atsushi Miyawaki

Atsushi Miyawaki obtained his PhD from Osaka University in 1991, after which he carried out postdoctoral research on intracellular signal transduction at the University of Tokyo. In 1995, he began to study fluorescent proteins and their application under Roger Y. Tsien at the University of California, San Diego in the United States. Since 1999, his group at the Laboratory for Cell Function Dynamics, part of the RIKEN Brain Science Institute, has been interested in the development of new bioimaging technologies, principally using fluorescent proteins.
