Sep. 13, 2013 Research Highlight Chemistry
Reviving iron catalysts for green chemistry
Iron nanoparticles protected by polymer resins can eliminate the need for costly and potentially toxic metal catalysts in an industrially important chemical reaction
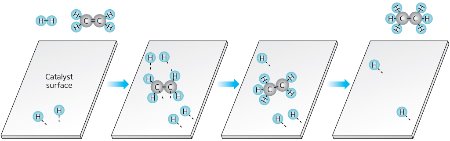 Figure 1: Example of a simple metal-catalyzed alkene hydrogenation reaction. When an alkene group bonds to a suitable metal surface, the carbon double bond (C=C) becomes a single bond (C–C) and two hydrogen atoms from the catalyst’s surface are transferred to the group to create a saturated alkane molecule. © 2013 Yoichi Yamada, RIKEN Center for Sustainable Science
Figure 1: Example of a simple metal-catalyzed alkene hydrogenation reaction. When an alkene group bonds to a suitable metal surface, the carbon double bond (C=C) becomes a single bond (C–C) and two hydrogen atoms from the catalyst’s surface are transferred to the group to create a saturated alkane molecule. © 2013 Yoichi Yamada, RIKEN Center for Sustainable Science
When the French emperor Napoleon III offered funds for a new butter substitute to feed his army and subjects in 1866, he unknowingly spurred development of the margarine industry, now valued in the billions of dollars. The first margarine consisted of churned beef tallow and milk, but French and German chemists soon found a way to turn inexpensive liquid vegetable oils into solid saturated fats through a process called catalytic alkene hydrogenation—a reaction that turns carbon double bonds into single bonds with the help of hydrogen gas and a metal catalyst (Fig. 1). Such hydrogenation processes also have wide applications beyond the food industry and are now one of the key technologies used in petrochemical refining, pharmaceutical synthesis and biofuel production.
The Achilles’ heel of hydrogenation chemistry remains its reliance on expensive platinum-series catalysts. The limited supply of these metals and their volatile pricing, as well as increasing awareness of their possible environmental toxicity, has prompted an intense search for alternative ways to add hydrogen to unsaturated chemical bonds. Yoichi Yamada and Yasuhiro Uozumi from the RIKEN Center for Sustainable Resource Science, in collaboration with researchers from McGill University in Canada and Japan’s Institute for Molecular Science, have now developed an efficient iron-based catalyst that promises to fundamentally change industrial hydrogenation processes1.
Getting the rust out
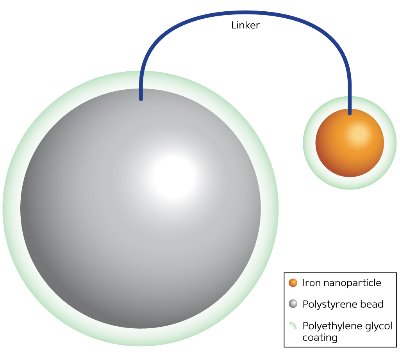 Figure 2: An iron nanoparticle with a polymer coating created through thermal decomposition of iron in the presence of polystyrene beads coated with polyethylene glycol. © 2013 Yoichi Yamada, RIKEN Center for Sustainable Science
Figure 2: An iron nanoparticle with a polymer coating created through thermal decomposition of iron in the presence of polystyrene beads coated with polyethylene glycol. © 2013 Yoichi Yamada, RIKEN Center for Sustainable Science
Iron (Fe) is the second most abundant metal in the Earth’s crust. It has an essential role in a multitude of biological processes and is used on large scales as a catalyst for ammonia fertilizer production. Iron can also catalyze hydrogenation reactions—but only under the right conditions. Iron catalysts require far higher gas pressures in comparison to the conventional metal catalysts, notes Uozumi, and the iron must remain in its pure metallic Fe(0) state. In fact, any contact with water or air during hydrogenation causes the iron catalyst to oxidize into iron oxide, which is not catalytic.
Because such conditions run counter to the demands of large-scale food manufacturing and other industries, iron has generally been ruled out as a hydrogenation catalyst. However, new research into iron-based nanoparticles has prompted chemists to take a second look at the potential of iron catalysts and their economic and environmental advantages. Such nanoparticles expose a significantly higher proportion of the active iron surfaces to chemical reactants than other structures and can also be attached to permanent substrates to boost catalyst recycling. Unfortunately, iron nanoparticles remain highly susceptible to oxidation and can even become explosive, necessitating special handling.
Yamada and Uozumi are specialists in the preparation of polymer resin coatings that allow metal particles to perform as catalysts in water. The pair met Chao-Jun Li and Audrey Moores, both experts in nanoscale catalysts, at the 2012 RIKEN–McGill University Scientific Workshop, and the group decided to combine their expertise to tackle the challenge of stabilizing iron nanoparticles with polymers.
According to Uozumi, optimal polymers for iron nanoparticles must be amphiphilic, containing both water-repelling and attracting components to protect the metal and at the same time ensure compatibility with aqueous mixtures. To achieve this goal, the researchers developed a synthetic procedure using polystyrene beads just 100 micrometers in diameter coated with polyethylene glycol. Heating an iron precursor in the presence of these beads results in thermal decomposition of the iron and the formation of iron nanoparticles coated with polyethylene glycol, which inhibits oxidation (Fig. 2). As part of the process, the protected nanoparticles and polystyrene beads are embedded in a resin that is sturdy and recyclable.
Going with the flow
The team tested the performance of the new catalyst under ‘flow chemistry’ reaction conditions in which reactants are continuously pumped past the iron nanoparticles inside a microreactor containing a series of interconnected millimeter-wide tubes. One of the primary benefits of this configuration is that the high hydrogen gas pressure needed for iron-catalyzed hydrogenation is localized into a small, heated compartment packed with polymer-supported nanoparticles. This greatly lowers the risks associated with high-pressure reactions and improves hydrogenation by exposing unsaturated molecules to high catalyst concentrations.
By studying a model organic hydrogenation reaction using their microreactor, the researchers discovered that the iron–polymer catalysts showed extraordinary tolerance toward water-based solvents. While other iron nanoparticles undergo significant activity loss in a 50:50 ethanol–water mixture, the hybrid iron–polymer materials achieved perfectly efficient hydrogenation in 99% pure water. Besides being one of the ‘greenest’ solvents for chemical transformations, water plays a critical role in enhancing the safety of iron nanoparticle catalysis by suppressing the possibility of explosive accidents, explains Uozumi.
The flow chemistry system also allowed the team to study multiple organic hydrogenation processes, which revealed that the new catalyst preferentially hydrogenates carbon bonds over other types of unsaturated chemical units. This behavior, which promises to be useful for industry, was put to the test by scaling up reactions to the multi-gram reactant conditions commonly seen in large reactors. The polymer-protected nanoparticles performed admirably and showed only a slight decrease in activity after many hours of use.
Not your average cup of tea
Intriguingly, the researchers also discovered that polyphenol compounds extracted from a cup of brewed black tea could also generate hybrid iron–polymer nanoparticles at room temperature. Although not as efficient as the thermal decomposition method, the team notes that small steps toward lowering costs and providing safer alternatives to conventional processes all help to make chemistry more sustainable. Currently, the researchers are investigating if other reactions can also overcome their dependence on costly metals with the help of amphiphilic polymers.
References
- 1. Hudson, R., Hamasaka, G., Osako, T., Yamada, Y. M. A., Li, C.-J., Uozumi, Y. & Moores, A. Highly efficient iron(0) nanoparticle-catalyzed hydrogenation in water in flow. Green Chemistry 15, 2141–2148 (2013). doi: 10.1039/c3gc40789f
About the Researcher
Yasuhiro Uozumi

Yasuhiro Uozumi graduated from Hokkaido University in 1984 and received his Doctor of Pharmacy degree from the same university. In 1994, he joined Columbia University in the United States as a research associate but soon returned to Japan to take up the position of lecturer at Kyoto University. Since 2000, Uozumi has served as professor at the Institute for Molecular Science and The Graduate University for Advanced Studies. In 2007, Uozumi became team leader at RIKEN, first heading the former Chemical Process Engineering Team and later, in 2010, the Nanocatalysis Research Team at the RIKEN Advanced Science Institute in Wako, which has since been reorganized into new centers. His current research explores novel catalytic reactions to develop chemical processes that are safe, simple and green.
