Sep. 27, 2013 Research Highlight Chemistry
One pot, many possibilities
A simple, one-pot method for the synthesis of a range of biologically useful fluorine-bearing amine molecules could aid drug development
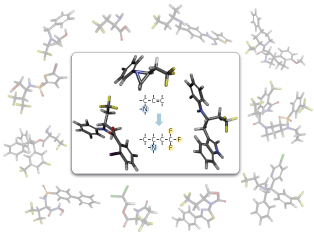 Figure 1: Three typical reaction products bearing the β-trifluoromethyl amine structure (center). The background shows a variety of bioactive compounds with the β-trifluoromethyl amine structure. © 2013 Mikiko Sodeoka, RIKEN Synthetic Organic Chemistry Laboratory
Figure 1: Three typical reaction products bearing the β-trifluoromethyl amine structure (center). The background shows a variety of bioactive compounds with the β-trifluoromethyl amine structure. © 2013 Mikiko Sodeoka, RIKEN Synthetic Organic Chemistry Laboratory
The trifluoromethyl group is present in many pharmaceutical and agrochemical products owing to its useful biological properties. A number of trifluoromethylated amine compounds display high bioactivity and are therefore of particular interest to medicinal chemists, but few synthetic methods for producing these compounds are known. Mikiko Sodeoka and colleagues from the RIKEN Synthetic Organic Chemistry Laboratory and RIKEN Center for Sustainable Resource Science have now developed a simple one-pot synthesis for a range of these important molecules1.
The synthetic method developed by Sodeoka’s team involves three different reactions, each starting with molecules containing both an amine group and an alkene. All are reacted with what is known as Togni’s reagent, which contains the trifluoromethyl group, CF3, in the presence of a catalytic amount of copper iodide. “The reaction uses the iodide salt of the inexpensive and highly abundant metal, copper, as a catalyst,” says Sodeoka.
In the first reaction, called ‘N-migratory oxytrifluoromethylation’, the trifluoromethyl group is added to one end of the starting molecule’s double-bonded carbon–carbon moiety (Fig. 1), and the molecule’s nitrogen atom migrates to the other end, to be replaced by carboxylic acid. This reaction establishes β-trifluoromethyl amine as a potentially useful intermediate molecule for the synthesis of various bioactive compounds.
When this reaction was stopped prematurely, the team discovered the presence of a small amount of a compound containing an aziridine group—a useful functional group due to its high reactivity. For their second reaction, the team therefore tweaked the reaction conditions to allow this compound to be produced at higher yields. In the third reaction, the aziridine compounds were functionalized by their reaction with a variety of nucleophiles in the reaction mixture. The whole process represents a one-pot, three-component coupling reaction.
“Our method provides highly functionalized amine derivatives from simple allylamine derivatives, which are commercially available or easy to prepare and handle,” says Sodeoka. “We believe that our new synthetic method provides medicinal chemists with the opportunity to examine more trifluoromethylated amine derivatives in their lead-optimization processes.” As Sodeoka explains, fluorine substitution near an amine center, such as by trifluoromethylation, is an important tool in the complex ‘lead optimization’ stage of drug development, where a potential drug compound is refined to produce a preclinical drug candidate. “Fluorine substitution not only lowers the amine’s basicity but also enhances its metabolic stability,” she notes.
“We are now planning to apply these reactions to the synthesis of bioactive molecules and will also try to develop new methods for the construction of other versatile structures containing a trifluoromethyl group,” adds Sodeoka.
References
- 1. Egami, H., Kawamura, S., Miyazaki, A. & Sodeoka, M. Trifluoromethylation reactions for the synthesis of β-trifluoromethylamines. Angewandte Chemie International Edition 52, 7841–7844 (2013). doi: 10.1002/anie.201303350
