Oct. 11, 2013 Research Highlight Chemistry
Building better molecules for bendable electronics
A sequence of ring-forming chemical reactions produces organic semiconductors ideal for flexible electronic device applications
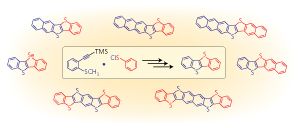 Figure 1: A synthetic procedure that selectively elongates the aromatic ring structures of organic semiconductors may prove useful for the development of flexible electronics. © 2013 American Chemical Society
Figure 1: A synthetic procedure that selectively elongates the aromatic ring structures of organic semiconductors may prove useful for the development of flexible electronics. © 2013 American Chemical Society
Organic semiconductors made from small aromatic molecules can be dissolved and screen-printed onto many substrates, including plastics, opening the path for ‘flexible’ electronic devices such as low-cost polymer solar cells. Kazuo Takimiya and colleagues from the Emergent Molecular Function Research Group at the RIKEN Center for Emergent Matter Science, in collaboration with researchers from Hiroshima University, have now developed a synthetic procedure that makes it easier to tailor the chemical structure of an important organic semiconductor1.
Takimiya and his team were studying molecules known as diacene-fused thienothiophenes when they discovered their new synthetic procedure. Diacene-fused thienothiophenes are composed of interlocking benzene and sulfur-containing aromatic rings and are more durable, and have higher charge carrier mobilities, than most other organic semiconductors. Although current schemes to make these compounds are relatively straightforward, they are also difficult to modify. Thus, chemists have a hard time producing derivatives based on this ring system with more desirable properties.
The researchers devised a creative synthesis that, instead of relying on bulky aromatic precursors, generates diacene-fused thienothiophenes from small molecules through two consecutive ring-forming reactions. First, they generated an active reagent called phenylsulfenyl chloride that joins to a benzene–acetylene molecule and transforms it into a three ring system. Then, they used selective carbon–hydrogen bond activation to set off a rare intramolecular coupling that produces a molecule with four fused rings known as benzothieno-benzothiophene (BTBT). Takimiya explains that this approach produces excellent yields and makes it possible to scrutinize numerous BTBT derivatives by making simple changes to the starting reagents.
Trials revealed that this technique was particularly useful for extending the ring structure of BTBT-type molecules (Fig. 1). For example, by substituting double- and triple-fused benzene molecules into the synthetic procedure, the team linearly constructed the BTBT substructure to form five, six and seven aromatic rings. Intriguingly, these new derivatives have an asymmetric structure that may dramatically improve their solubility—an important processing feature for printed electronics and one that is difficult to achieve using existing synthetic techniques.
Lengthening the BTBT framework to an eight-ringed symmetric structure also yielded a potent new organic semiconductor with excellent thermal stability and a charge carrier mobility five times higher than that of BTBT. “This mobility is among the highest recorded for thin film organic field-effect transistors, meaning that this molecule could be a candidate for real flexible electronics applications in the future” says Takimiya.
References
- 1. Mori, T., Nishimura, T., Yamamoto, T., Doi, I., Miyazaki, E., Osaka, I. & Takimiya, K. Consecutive thiophene-annulation approach to π-extended thienoacene-based organic semiconductors with [1]benzothieno[3,2-b][1]benzothiophene (BTBT) substructure. Journal of the American Chemical Society 135, 13900–13913 (2013). doi: 10.1021/ja406257u
