May 2, 2014 Research Highlight Biology
Unraveling a highly specific protein modification
A pattern of protein modifications specific to the brain is driven by the tissue-specific regulation of multiple enzymes
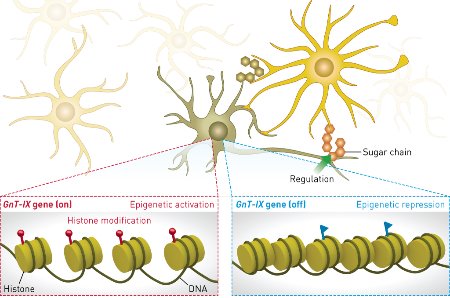 Figure 1: In the brain, epigenetic factors can increase expression of the enzyme GnT-IX to produce a specific glycosylation pattern (left), or block GnT-IX expression to prevent the same glycosylation process (right). © 2014 Yasuhiko Kizuka, RIKEN–Max Planck Joint Research Center for Systems Chemical Biology
Figure 1: In the brain, epigenetic factors can increase expression of the enzyme GnT-IX to produce a specific glycosylation pattern (left), or block GnT-IX expression to prevent the same glycosylation process (right). © 2014 Yasuhiko Kizuka, RIKEN–Max Planck Joint Research Center for Systems Chemical Biology
Many human proteins are modified by the attachment of short sugar chains called glycans. The patterns of attachment, or glycosylations, are known to be specific to organs, tissues and cell types, but the mechanisms regulating these patterns have been unclear. Naoyuki Taniguchi, Yasuhiko Kizuka and colleagues from the RIKEN–Max Planck Joint Research Center for Systems Chemical Biology have now clarified one such mechanism in the brain1.
Glycosylation is mediated by glycosyltransferase enzymes. The researchers studied one of these enzymes called N-acetylglucosaminyltransferase-IX (GnT-IX), which is expressed only in the brain and is involved in demyelination—degradation of the insulation around neurons that helps them to conduct electrical signals. Understanding the regulation of GnT-IX expression could help in developing better knowledge of diseases such as multiple sclerosis.
As Kizuka explains, a closer look at the process regulating GnT-IX expression could also provide broader insight into glycosylation. “Expression of each glycosyltransferase enzyme in a specific tissue contributes to the phenomenon of tissue-specific glycosylation,” he says. “Unveiling the mechanism of how GnT-IX is present only in the brain could lead to a general understanding of how sugar chains are formed in a tissue-specific manner.”
The team focused on a process known as epigenetics, in which gene expression is altered over time by modification of DNA and the histone proteins they are wrapped around. By manipulating expression of epigenetic factors in cultured brain cells and measuring the effect on GnT-IX expression, the researchers found that an enzyme called histone deacetylase 11 (HDAC11), which modifies histones near the GnT-IX gene, blocks expression of the GnT-IX enzyme. In contrast, enzymes called ten eleven translocase 3 (TET3) and O-GlcNAc transferase (OGT) increase expression of GnT-IX by recruiting a protein called NeuroD1, which triggers expression of the GnT-IX gene. The expression of enzymes similar to GnT-IX was unaffected, showing that this control mechanism is highly specific (Fig. 1).
“For the first time, we revealed the epigenetic mechanism underlying tissue-specific glycosylation,” says Kizuka. “I expect that similar epigenetic mechanisms regulate other glycosylation-related genes, and analyzing the regulation of these genes will promote our understanding of glycosylation.”
Kizuka also notes that it could be this epigenetic mechanism of GnT-IX regulation that goes wrong in the development of demyelination diseases. If similar mechanisms operate for other enzymes, there could be wider implications. “Defects in glycosylation often lead to severe disorders,” he says. “Some of these diseases might be triggered by epigenetic dysregulation of glycosylation-related genes.”
References
- 1. Kizuka, Y., Kitazume, S., Okahara, K., Villagra, A., Sotomayor, E. M. & Taniguchi, N. Epigenetic regulation of a brain-specific glycosyltransferase N-acetylglucosaminyltransferase-IX (GnT-IX) by specific chromatin modifiers. The Journal of Biological Chemistry 289, 11253–11261 (2014). doi: 10.1074/jbc.M114.554311
