Apr. 3, 2015 Research Highlight Chemistry
One at a time, please
Polymers held together by multiple weak intermolecular interactions but with tight control over the number of monomer units in each chain have been prepared for the first time
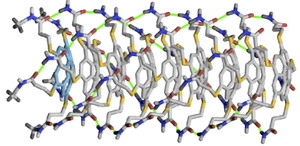 Figure 1: A molecular model of the supramolecular corannulene polymer showing the stacked-bowl structure of the polymer with the initiator highlighted at the left end. Reproduced, with permission, from Ref. 1 © 2015 J. Kang et al.
Figure 1: A molecular model of the supramolecular corannulene polymer showing the stacked-bowl structure of the polymer with the initiator highlighted at the left end. Reproduced, with permission, from Ref. 1 © 2015 J. Kang et al.
The first example of a supramolecular polymer that grows by the addition of one monomer at a time has been reported by researchers from the RIKEN Center for Emergent Matter Science1. This process, and the understanding of the particular properties of the monomer that drive it, could lead to the development of precision polymer transistors and other applications requiring tightly controlled polymer dispersity.
Supramolecular polymers consist of monomer units held together by relatively weak intermolecular forces. As their electronic and physical properties are usually highly dependent on the number of monomers in the chain, controlling the polymer growth process so that all the polymer chains are of similar length—producing polymers with low ‘dispersity’—has been a long-term goal of both scientists and engineers. Yet the goal has often been thought to be impossible because short polymers would sometimes join together, resulting in step-growth polymerization rather than the desired monomer-by-monomer chain-growth polymerization.
The research team, led by RIKEN researchers Takuzo Aida and Daigo Miyajima, has been working for over a decade on monomers based on corannulene—a polycyclic aromatic hydrocarbon with a bowl-like structure that can flip inside-out to form a mirror image of itself. In the polymer, the corannulene monomer units stack together like soup plates and are held in place by hydrogen bonds formed between amides that decorate the edges of the bowl.
“In more than ten years of research, this is the first monomer that we have found that did not spontaneously polymerize at room temperature,” explains Miyajima. “We attribute this to the formation of hydrogen bonds between amides in the monomer.”
These intramolecular hydrogen bonds tie the monomers up in unreactive, ball-like structures. The addition of a small amount of a special monomer that cannot form these intramolecular hydrogen bonds can initiate polymerization, but because each ball-like monomer must open up before adding to the chain, monomers can only be added to the chain one at a time. The ‘stack of soup plates’ structure of the growing polymers, with one end blocked by the initiator (Fig. 1), inhibits step-growth polymerization and provides tight dispersity control.
The resultant polymers have a nanofibrous structure that can be seen using atomic force microscopy. “The corannulene structure is known to have semiconducting properties,” says Miyajima, “so we hope to be able to fabricate very small transistors using these polymers. We are also working on developing the concept of metastable monomers for the production of other dispersity-controlled supramolecular polymerizations.”
References
- 1. Kang, J., Miyajima, D., Mori, T., Inoue, Y., Itoh, Y. & Aida, T. A rational strategy for the realization of chain-growth supramolecular polymerization. Science 347, 646–651 (2015). doi: 10.1126/science.aaa4249
