Aug. 19, 2016 Research Highlight Chemistry
A radical route to fluorine-containing molecules
Two new strategies have been developed to incorporate fluorine atoms into potential drugs
Free-radical chemistry offers a surprisingly efficient way to add fluorine atoms to candidate drug molecules, RIKEN researchers have shown. Radicals are highly reactive chemical species, but the team tamed this unruly behavior using a copper catalyst to smoothly and selectively add fluorine-containing units to a range of molecular reaction partners1.
Adding fluorine is a proven way to improve the performance of potential therapeutic drugs. Attaching a three-fluorine unit called a trifluoromethyl group can make a molecule more soluble in fat, which can help it move through the body to reach its target. In the same vein, fluorine can also improve the efficacy of agrochemicals used to treat crops.
However, the chemical protocols currently used to introduce fluorine leave a lot to be desired. One of the best methods uses a trifluoromethylating agent known as Togni’s reagent, but it is time consuming and costly to prepare the reagent, limiting its appeal for industry.
Mikiko Sodeoka and Shintaro Kawamura from the RIKEN Center for Sustainable Resource Science and the Synthetic Organic Chemistry Laboratory at RIKEN have developed a method that instead uses an inexpensive and easily produced perfluoroalkylating agent, called perfluoro acid anhydrides including trifluoroacetic anhydride.
In a reaction flask, this material is decomposed to release trifluoromethyl radicals, which then attach to partner molecules containing a carbon–carbon double bond. When the team first tried the reaction, a complex mixture of products was formed. “This result indicated that we had to improve not the reactivity but the product selectivity,” Sodeoka says.
When the trifluoromethyl radical attaches to a partner molecule, the partner molecule also briefly becomes a highly reactive alkyl radical. The team suspected that adding a metal catalyst to the reaction mixture would transform the alkyl radical into a semi-stable intermediate. “After screening the metals, a copper catalyst was found to be the best,” Sodeoka adds.
By using the copper-catalyzed protocol, the team was able to attach the trifluoromethyl and other perfluoroakyl groups to a range of starting molecules containing a carbon–carbon double bond. They also discovered that, for certain starting compounds with an aromatic ring, they could produce useful perfluoroalkylated cyclic products without employing a metal catalyst.
“Although we and other groups had reported useful alkene trifluoromethylation reactions, the expensive and potentially hazardous trifluoromethyl source was a problem,” Sodeoka says. The latest study shows how to control safe, inexpensive perfluoroacid anhydrides instead, she says. “Various related transformations based on the principles found in this work will now be developed—not only by us, but also by many other research groups.”
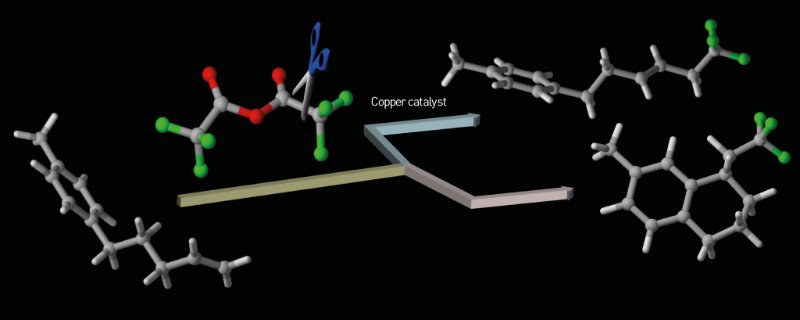 Figure 1: Trifluoroacetic anhydride (red and green molecule) and a copper catalyst are an effective combination for adding fluorine atoms (green) to a molecule. © 2016 Shintaro Kawamura, RIKEN Center for Sustainable Resource Science and RIKEN Synthetic Organic Chemistry Laboratory
Figure 1: Trifluoroacetic anhydride (red and green molecule) and a copper catalyst are an effective combination for adding fluorine atoms (green) to a molecule. © 2016 Shintaro Kawamura, RIKEN Center for Sustainable Resource Science and RIKEN Synthetic Organic Chemistry Laboratory
Related contents
References
- 1. Kawamura, S. & Sodeoka, M. Perfluoroalkylation of unactivated alkenes with acid anhydrides as the perfluoroalkyl source. Angewandte Chemie International Edition 55, 8740–8743 (2016). doi: 10.1002/anie.201604127
