Apr. 10, 2015 Research Highlight Biology
Protein contortions keep cells together
Giant cell adhesion molecules squeeze into the tiny spaces between cells by kinking their structure
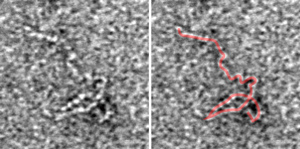 Figure 1: Transmission electron microscopy image of the kinked structure of Fat4 (left). The structure is highlighted in red on the right. Reproduced from Ref. 1 © 2014 Y. Tsukasaki et al.
Figure 1: Transmission electron microscopy image of the kinked structure of Fat4 (left). The structure is highlighted in red on the right. Reproduced from Ref. 1 © 2014 Y. Tsukasaki et al.
A high-resolution electron microscopy study by the RIKEN Center for Developmental Biology has revealed that bulky cell adhesion molecules kink to fit into the narrow spaces between cells1. The finding helps explain how these large supramolecules maintain cell adhesion, and it could assist in identifying the causes of certain developmental diseases.
Cell-to-cell adhesion is essential for maintaining multicellular structures. It involves the action of adhesion proteins called cadherins that mediate close cell-to-cell contacts by binding to other cadherins across narrow extracellular spaces. However, some mammalian cadherins, including the proteins called Fat and Dachsous, have very large extracellular domains, and it was unclear how such large molecules could fit into the tight junctional spaces to mediate cell adhesion.
Led by RIKEN cell biologist Masatoshi Takeichi, the research team examined the structure of the portion of Fat and Dachsous that sticks out between cells by purifying the proteins and comparing their structures with those of the much smaller adhesion protein, E-cadherin. Using transmission electron microscopy to directly visualize the protein shape, they found that E-cadherin has a slightly curved shape, while the larger cadherin family members contained both curves and tight hairpin kinks (Fig. 1). It is this kinking that makes it possible for the large cadherins to adapt to tight intercellular spaces.
Kinked cadherins had previously been observed in flies in association with a lack of a calcium-binding motif (CBM) in those proteins. The researchers therefore investigated whether mammalian Fat and Dachsous kinked for the same reason. They found that the CBM of those mammalian proteins contained particular mutations that were not seen in non-kinked extracellular domains, such as those of E-cadherin. Introduction of the CBM sequences of Fat and Dachsous into E-cadherin resulted in kinking of the E-cadherin protein, confirming the role of these mutated CBMs in protein kinking.
The researchers then expressed Fat and Dachsous in kidney cells that do not normally express these proteins. Without these cadherins, the kidney cells formed ordinary contact structures. However, kidney cells expressing both of these cadherins formed extra junctions about 50 nanometers wide at abnormal locations on the cell boundaries.
“Mutations in the Fat4 or Dachsous1 genes cause a human disease called Van Maldergem syndrome, which is characterized by intellectual disability, hearing loss, craniofacial and limb malformations,” says Takeichi. “The results of our study could help identify the mechanisms by which the corresponding adhesion molecules lose their functions in such diseases,” he explains.
References
- 1. Tsukasaki, Y., Miyazaki, N., Matsumoto, A., Nagae, S., Yonemura, S., Tanoue, T., Iwasaki, K., Takeichi, M. Giant cadherins Fat and Dachsous self-bend to organize properly spaced intercellular junctions. Proceedings of the National Academy of Sciences USA 111, 16011–16016 (2014). doi: 10.1073/pnas.1418990111
