Feb. 26, 2015
SARC bodies: markers of cellular stress needed for muscle cell differentiation
During skeletal muscle development, muscle precursor cells called myoblasts begin to express muscle-specific genes and actually fuse together to form the multinuclear cells characteristic of muscle fibers, existing in essence as a single cell with up to thousands of nuclei. The fusion process is driven by stress in the endoplasmic reticulum (ER)—a manufacturing and storage organelle inside cells. This stress leads to the death of some cells but helps the remaining cells to fuse. Recently, researchers at the RIKEN Molecular Membrane Biology laboratory determined that this process results specifically from stress caused by a transient depletion of calcium in the ER manifested by the formation of ER-derived globular structures that the authors have termed stress-activated response to calcium depletion (SARC) bodies.
SARC bodies were first observed by the team after labeling myoblast cells with ER-specific stain and placing them in differentiation medium. After several days, they observed up to 20 SARC bodies in each cell, but this number decreased gradually during fusion until none remained. Their appearance coincided with the expression of protein markers for ER stress. To determine the nature of this stress, the researchers artificially induced ER stress in non-differentiating cells through several means, and found that the globular structures only formed as a result of calcium depletion-induced ER stress (hence the name, SARC body).
Next, they observed the distribution of GFP-STIM1 in differentiating myoblasts, and found that the distribution pattern was characteristic of ER undergoing calcium depletion, confirming that calcium depletion occurs in differentiating myoblasts, which then leads to the formation of SARC bodies.
A closer look at the structure of SARC bodies revealed that they were composed of 4 to 10 pseudoconcentric sheets that were likely derived from rough ER—the types of endoplasmic reticulum where proteins are made—and that in cross-section, the rings were evenly spaced and did not touch or coil.
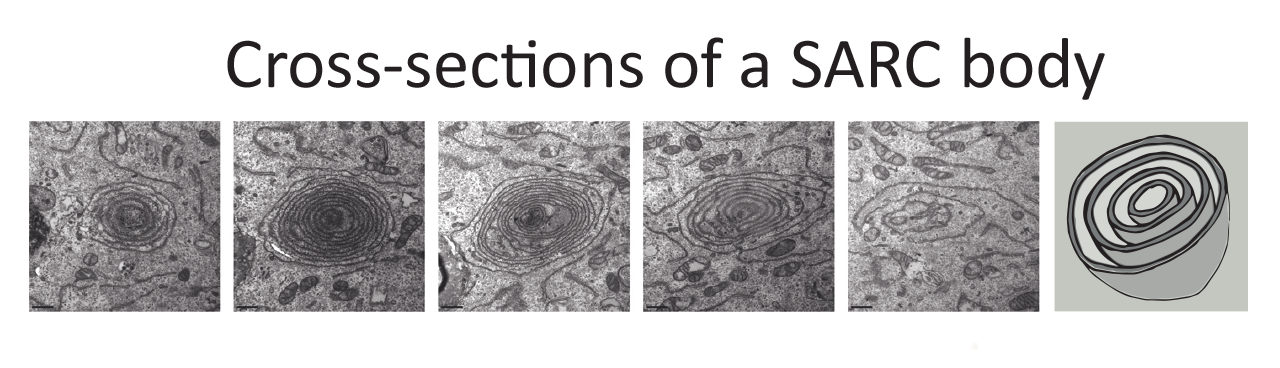
Five horizontal cross-sections of a SARC body at different vertical positions followed by a schematic depicting a 3D model
When induced using differentiation medium, the inside spaces of the SARC bodies—their lumens—appeared narrower than those of most rough ER. Further testing showed a similar reduction in luminal widths when calcium influx into the ER was inhibited using thapsigargin. In contrast, introducing ER stress by other means did not make the lumens narrower.
Next, the researchers showed that calcium depletion is necessary for muscle differentiation. They placed myoblasts in a differentiation medium that contained ER calcium-channel inhibitors and found that (1) markers for ER stress were reduced, (2) SARC bodies were found in less than 5% of the cells, (3) skeletal myosin characteristic of muscle cells was absent, and (4) the myoblasts did not fuse. In vivo examination showed that SARC bodies appeared in the developing embryo, and were observed in less-differentiated cells, consistent with the initial in vitro experiments.
