Dec. 15, 2017 Research Highlight Biology
How bacteria avoid poisoning themselves
Scientists have found how bacteria rid themselves of a toxic metabolic by-product—a finding that could aid the development of antimicrobial drugs
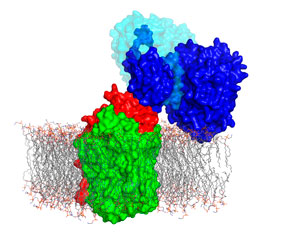 Figure 1: Nitric oxide-producing nitrite reductase (dark blue) and nitric oxide-decomposing nitric oxide reductase (red and green) form a complex that prevents the pathogenic bacteria Pseudomonas aeruginosa from poisoning itself with nitric oxide. © 2017 RIKEN Biometal Science Laboratory
Figure 1: Nitric oxide-producing nitrite reductase (dark blue) and nitric oxide-decomposing nitric oxide reductase (red and green) form a complex that prevents the pathogenic bacteria Pseudomonas aeruginosa from poisoning itself with nitric oxide. © 2017 RIKEN Biometal Science Laboratory
RIKEN researchers have discovered how bacteria that produce the toxic gas nitric oxide avoid poisoning themselves1. They found that proteins that generate and degrade nitric oxide form a single complex, allowing bacteria to neutralize nitric oxide immediately after it is produced.
In low-oxygen environments, some bacteria can produce energy using nitrate instead of oxygen. These include some pathogenic bacteria, such as Pseudomonas aeruginosa, a notorious cause of opportunistic infections in hospital patients.
The process by which these bacteria generate energy from nitrate, known as denitrification, produces nitric oxide as a toxic by-product. Although researchers knew that denitrifying bacteria need to tightly control nitric oxide production, they did not know how this was controlled.
In P. aeruginosa, nitric oxide is produced by the enzyme nitrite reductase (NiR) and decomposed by the enzyme nitric oxide reductase (NOR). Takehiko Tosha at the RIKEN Biometal Science Laboratory and his colleagues conjectured that these two proteins might combine to form a single structure. To test this idea, they isolated the two enzymes and examined their structures using x-ray diffraction. They found that the proteins did indeed fit together like puzzle pieces.
“Nitric oxide-producing NiR and nitric oxide-decomposing NOR form a complex to prevent cytotoxic nitric oxide diffusing into the cellular environment,” says Tosha. Locating nitric oxide production and disposal in a single unit effectively prevents leakage of the toxic by-product (Fig. 1).
 Takehiko Tosha and co-workers have found that the proteins that produce and degrade toxic nitric oxide in denitrifying bacteria form a complex. © 2017 RIKEN
Takehiko Tosha and co-workers have found that the proteins that produce and degrade toxic nitric oxide in denitrifying bacteria form a complex. © 2017 RIKEN
The researchers next investigated how the complex is bound together as well as the consequences of breaking it apart. Using computer simulations, they determined which parts of the protein form the key chemical associations that bind NiR and NOR. They then created mutant strains of P. aeruginosain which these key interactions were disrupted, impeding complex formation. The mutant strains showed up to a 30 per cent reduction in growth. Inhibiting complex formation hampered the bacteria’s ability to neutralize nitric oxide, causing them to poison themselves.
These results could have potent medical applications. “Denitrification is crucial for some pathogenic bacteria including P. aeruginosa to survive under low-oxygen conditions, such as inside biofilms, during infection,” explains Tosha. “Since we found that the interaction between NiR and NOR contributes to denitrification-dependent anaerobic growth of P. aeruginosa, compounds that inhibit this interaction might work as antimicrobial drugs.”
Tosha and his colleagues next plan to investigate whether the proteins responsible for processing nitrite, another toxic intermediate in this pathway, also form a complex that could be disrupted.
Related contents
- Revealing a pollutant’s Achilles’ heel
- New study sheds light on evolutionary origin of oxygen-based cellular respiration
- The roots of respiration
References
- 1. Terasaka, E., Yamada, K., Wang, P.-H., Hosokawa, K., Yamagiwa, R., Matsumoto, K., Ishii, S., Mori, T., Yagi, K., Sawai, H. et al. Dynamics of nitric oxide controlled by protein complex in bacterial system. Proceedings of the National Academy of Sciences USA 114, 9888–9893 (2017). doi: 10.1073/pnas.1621301114
