May 17, 2022 Research Highlight Biology
Mouse stem cells for primitive endoderm established
The third type of stem cells that make up the precursors of mouse embryos has been established for the first time
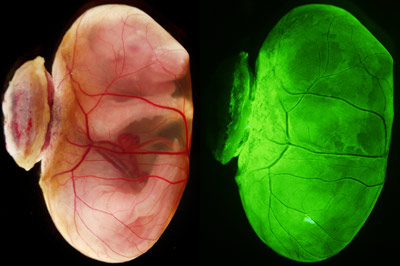 Figure 1: Bright-field microscopy image (left) and fluorescence microscopy image (right) of an E18.5 embryo with a yolk sac. When blastocysts that were depleted in primitive endoderm cells and injected with primitive endoderm stem cells were implanted in the uteri, they developed living offspring with yolk sacs (structure encasing the embryo), just like wild-type blastocysts. From Ref. 1. Reprinted with permission from AAAS.
Figure 1: Bright-field microscopy image (left) and fluorescence microscopy image (right) of an E18.5 embryo with a yolk sac. When blastocysts that were depleted in primitive endoderm cells and injected with primitive endoderm stem cells were implanted in the uteri, they developed living offspring with yolk sacs (structure encasing the embryo), just like wild-type blastocysts. From Ref. 1. Reprinted with permission from AAAS.
Stem cells that give rise to the mouse yolk sac have been isolated and cultured in the lab for the first time by RIKEN researchers, raising the possibility of artificially creating mouse embryos from stem cells in the future1.
After fusing with a sperm, an egg develops into an early embryo called a blastocyst. It consists of dozens of cells of just three types: epiblast cells (forming the embryo), trophoblast cells (forming the placenta) and primitive endoderm cells (producing major parts of the yolk sac).
Stem cells have been isolated for both epiblast and trophoblast cells, but until now no-one had succeeded in producing stem cells for primitive endoderm cells.
Now, Yasuhide Ohinata of the RIKEN Center for Integrative Medical Sciences and co-workers have established primitive endoderm stem cells in mice, which form the yolk sac that sustains the developing blastocyst until the placenta takes over this role.
They achieved this by placing mouse blastocysts in various cultures and determining the conditions under which primitive endoderm stem cells flourished. Specifically, they perturbed key functional signaling pathways in the blastocysts by using recombinant proteins and small molecules, then observed the resulting cells.
 Yasuhide Ohinata (right) and Haruhiko Koseki (left) of the RIKEN Center for Integrative Medical Sciences and co-workers have established primitive endoderm stem cells in mice. © 2022 RIKEN
Yasuhide Ohinata (right) and Haruhiko Koseki (left) of the RIKEN Center for Integrative Medical Sciences and co-workers have established primitive endoderm stem cells in mice. © 2022 RIKEN
The researchers confirmed that these cells fully complement fetal development of primitive-endoderm-cells-depleted blastocysts in chimeras. These blastocysts developed into normal offspring after transfer into uteri.
This demonstration in living mice wasn’t a trivial step. “There’s a big leap between in vitro culture and in vivo function, as it’s impossible to know whether established stem cells will function in vivo without transplanting them,” says Ohinata. “We were fortunate in that we were able to achieve the expected results in vivo.”
The team eventually hopes to artificially create embryos. “Our ultimate goal is to reconstitute embryos that can develop from stem cells alone,” says Ohinata. “Primitive endoderm stem cells are essential for this, which is why we devoted so much effort to establishing a generally overlooked stem-cell type.”
The researchers combined the three types of stem cells to generate embryo-like structures in vitro. When they implanted these in uteri, they formed descendants with yolk-sac-like structures but didn’t develop into normal embryos. “While these results represent a major step forward in artificial embryo reconstitution, we will continue to pursue research to mimic embryos more precisely using stem cells,” says Ohinata.
Ohinata notes that this achievement may not be directly applicable to people because of the differences between embryonic development in mice and humans, but he adds: “To understand human and mouse development in an integrated manner, we’re conducting research using pigs, which are thought to retain human-type developmental mechanisms.”
Related contents
- Implantable 3D blastocyst-like embryonic structure generated from mouse stem cells
- mRNA degradation induced by fluid flow breaks left–right symmetry in vertebrates
- How an embryo stops being an egg
Rate this article
Reference
- 1. Ohinata, Y., Endo, T. A., Sugishita, H., Watanabe, T., Iizuka, Y., Kawamoto, Y., Saraya, A., Kumon, M., Koseki, Y., Kondo, T. et al. Establishment of mouse stem cells that can recapitulate the developmental potential of primitive endoderm. Science 375, 574–578 (2022). doi: 10.1126/science.aay3325
