Feb. 9, 2024 Research Highlight Chemistry
Helium droplets capture double water structure
A water ion predicted by calculations has been spotted in the lab for the first time
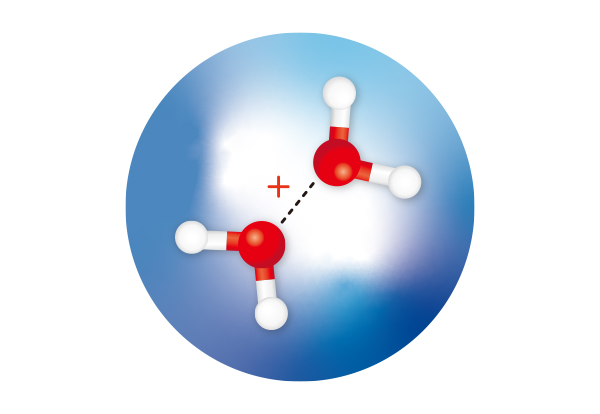
Figure 1: RIKEN researchers have isolated and observed water dimer cations (red spheres: oxygen atoms; white spheres: hydrogen atoms) within helium nanodroplets (large blue sphere) and determined the structures of the isomers. Adapted from Ref. 1 and licensed under CC BY 4.0 ©2024 A. Iguchi et al.
An elusive structure involving two water molecules, which had been predicted but never observed, has been isolated by RIKEN chemists1. This finding could have implications for a wide range of fields ranging from astrochemistry to corrosion of metals.
An energetic particle or photon can knock out an electron from a water molecule, creating a positive ion (cation; H2O+) and an electron. This ionization of water can trigger a cascade of other reactions with nearby molecules.
Water ionization plays important roles in biological processes and radiation chemistry as well as promotes corrosion at interfaces between water and metals. How the ionization of water proceeds is thus a critical question for physical chemists.
Calculations predict that following the ionization of a water molecule, two isomers of a positively charged ion of a water dimer—two water molecules loosely connected by a weak bond—will form rapidly. One isomer (H3O+.OH) has been observed and is formed when a proton is transferred from one water molecule to another. The other isomer has a half-bond (or hemibonded) structure (H2O.OH2)+, but it has never been isolated or confirmed by spectroscopic measurements. Calculations suggest that it has a higher energy than the proton-transfer dimer.
Now, Susumu Kuma of the RIKEN Atomic, Molecular and Optical Physics Laboratory and his co-workers have isolated both water dimer ions by trapping them in tiny droplets of cold helium. They also used infrared spectroscopy to determine their structures.
Kuma and co-workers used an ultracold environment to make the isomers. The water molecules in the helium droplets cooled rapidly as helium atoms evaporated from the droplet’s surface. This process formed the metastable hemibonded isomer because of its very fast stabilization within the cold droplets.
Kuma and his team then probed the co-existence of the two isomers using computational and spectroscopic methods. The spectroscopic signatures of the molecular ions were almost identical to those of bare ions, without helium surrounding them. “This finding indicates that we can directly compare measurements on the bare ions with results from quantum-chemical calculations,” says Kuma. “This greatly facilitates the structural analysis of the dimers.”
The finding will help spawn further studies in this field, Kuma predicts. “The discovery of the hemi-bonded water cations will promote further studies of primary events that are important for understanding the radiation chemistry of water,” he says.
Kuma’s team intends to look for other structures that have yet to be observed. “We plan to extend the size of the water complex cations in the helium droplets,” Kuma says. “We expect to find previously unobserved, but important, chemical structures in the spectra.”

Susumu Kuma (second from left) with co-workers Andrey Vilesov (far left), Arisa Iguchi (second from right) and Amandeep Singh (far right) during an experiment at the University of Southern California in the United States. ©2024 RIKEN
Related contents
- Water surface molecules lose energy through rotation of free OH group
- Scanning tunneling microscopy reveals molecular dissociation induced by localized surface plasmons
Rate this article
Reference
- 1. Iguchi, A., Singh, A., Bergmeister, S., Azhagesan, A. A., Mizuse, K., Fujii, A., Tanuma, H., Azuma, T., Scheier, P., Kuma, S. et al. Isolation and infrared spectroscopic characterization of hemibonded water dimer cation in superfluid helium nanodroplets. The Journal of Physical Chemistry Letters 14, 8199–8204 (2023). doi: 10.1021/acs.jpclett.3c02150
