Sep. 22, 2017 Research Highlight Chemistry
Useful new supramolecular polymer responds to heat and alcohol
An intriguing new plastic that forms by either cooling or heating its components in alcohol promises numerous applications
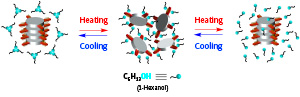 Figure 1: At moderate temperatures (middle image), monomers of a new supramolecular polymer are prevented from linking together by alcohol molecules binding to their side arms. But on either heating or cooling, the alcohol molecules detach from the monomers, allowing them to join together and form the polymer. Adapted by permission from Macmillan Publishers Ltd: Nature Chemistry (Ref. 1), copyright (2017)
Figure 1: At moderate temperatures (middle image), monomers of a new supramolecular polymer are prevented from linking together by alcohol molecules binding to their side arms. But on either heating or cooling, the alcohol molecules detach from the monomers, allowing them to join together and form the polymer. Adapted by permission from Macmillan Publishers Ltd: Nature Chemistry (Ref. 1), copyright (2017)
Engine oils and smart tinted windows are just two of the many potential applications of an unusual new type of plastic developed by researchers at RIKEN.
The downsides of most conventional plastics and polymers are their low biodegradability and extreme persistence in the environment for decades. This is because their building blocks, or monomers, are held together by strong covalent bonds.
Recently, attention has turned to a new kind of polymer known as supramolecular polymers. These polymers have weaker hydrogen bonds and hence are much easier to degrade into their constituent monomers—often gently heating them to about 80 degrees Celsius is sufficient. This makes them easier to reuse and recycle, but it also means that supramolecular polymers cannot be used in applications involving high temperatures.
Now, Kotagiri Venkata Rao, Daigo Miyajima, Atsuko Nihonyanagi and Takuzo Aida, all at the RIKEN Center for Emergent Matter Science, have succeeded in engineering a supramolecular polymer that is able to withstand temperatures of more than 110 degrees Celsius1. The key to this stability lies in the eight amide groups (–CONH2) on the four side arms of the monomer. The weak hydrogen bonds between these units are enough to allow the monomers to form a stable structure when joined together to form the polymer.
Most excitingly, the team’s supramolecular polymer exhibits highly unusual behavior when combined with an alcohol. At a temperature of about 50 degrees Celsius, the alcohol molecules form hydrogen bonds with the monomer molecules, preventing them from joining together to form the supramolecular polymer. But either cooling or heating the mixture causes the alcohol molecules to disconnect from the monomers, freeing them up to form the polymer (Fig. 1).
Miyajima says there are lots of potential applications for temperature-dependent plastics. “Our polymer could be used to develop smart windows that let little light through at low and high temperatures, but become transparent at moderate temperatures,” he explains. The team is also exploring using the polymer in high-performance engine oils whose viscosity increases on heating.
The discovery opens up a new approach for designing polymers. “Previously, various supramolecular polymers have been synthesized by focusing mainly on the molecular designs of their monomers,” says Miyajima. “Our work has shown the potential of using small additives such as alcohols to expand applications of supramolecular polymers. Using this approach, we are confident that the current limitations of supramolecular polymers for applications can be overcome.”
Related contents
References
- 1. Rao, K. V., Miyajima, D., Nihonyanagi, A. & Aida, T. Thermally bisignate supramolecular polymerization. Nature Chemistry advance online publication, June 2017 doi: 10.1038/nchem.2812
