Nov. 29, 2019 Research Highlight Chemistry
Protein changes precede photoisomerization of retinal chromophore
An unexpected finding shows that the protein component of bacteriorhodopsin reacts first to light
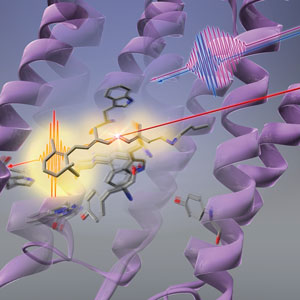 Figure 1: RIKEN researchers have found that light (long red line) first causes the shape of the protein (purple twirls) to change before the retinal chromophore (stick-like structure) in bacteriorhodopsin undergoes photoisomerization. © 2019 RIKEN Center for Advanced Photonics
Figure 1: RIKEN researchers have found that light (long red line) first causes the shape of the protein (purple twirls) to change before the retinal chromophore (stick-like structure) in bacteriorhodopsin undergoes photoisomerization. © 2019 RIKEN Center for Advanced Photonics
The sequence of changes that light triggers in a bacterial photoreceptor starts with its protein scaffolding rather than the light-absorbing chromophore, an all-RIKEN team has shown1. This finding goes against conventional wisdom and sheds new light on how photoreceptors can convert light into chemical energy so efficiently.
Many bacteria use special light-sensitive molecules known as photoreceptor proteins to turn light into chemical energy, which they use to initiate various biological functions.
Scientists have long wanted to know how bacterial photoreceptors are so efficient in converting light. “One of the fundamental questions is how these biomolecules realize such high-efficiency, low-energy photoreactions,” says Tahei Tahara. “This has been a long-standing question.” One motivation for uncovering the mechanism of these photoreceptors is that it could inform efforts to develop artificial versions of these molecules.
The most well-studied bacterial photoreceptor, bacteriorhodopsin, contains a retinal chromophore, which changes shape when it absorbs a photon of yellow light. This configuration change sets off a series of structural changes in bacteriorhodopsin that enables it to pump protons.
Interestingly, when the retinal chromophore of bacteriorhodopsin is placed in solution, its light-conversion efficiency is three times lower than when it is nestled within the protein structure of bacteriorhodopsin. This clearly indicates that the protein plays an important role in aiding the conversion of light into chemical energy.
 Tahei Tahara (center, wearing dark jacket) and his group have shown that protein dynamics precede the photoisomerization of the retinal chromophore in bacteriorhodopsin. © 2019 RIKEN
Tahei Tahara (center, wearing dark jacket) and his group have shown that protein dynamics precede the photoisomerization of the retinal chromophore in bacteriorhodopsin. © 2019 RIKEN
The conformational change of the retinal chromophore was assumed to be the first response of bacteriorhodopsin to light. But Tahara and his co-workers at the RIKEN Molecular Spectroscopy Laboratory and the RIKEN Center for Advanced Photonics have now discovered that there is a step that precedes it—the protein that cradles the retinal chromophore first alters its shape in response to light. This change in the protein could help the retinal chromophore use light efficiently.
The team took a spectroscopic technique known as femtosecond stimulated Raman spectroscopy, which can observe processes that occur faster than a picosecond (1 picosecond = 10−12 seconds), and extended it to the deep ultraviolet region. This allowed them to look at the protein part of bacteriorhodopsin (Fig. 1).
This discovery came as a surprise to Tahara. “I didn’t expect that the protein would change shape before chromophore isomerization, but when I saw the experimental results I thought ‘Wow, it’s actually the case’,” he says. “It was most surprising, and we were very excited.”
While the team looked at bacteriorhodopsin in this study, they anticipate that the same effect could well occur in other rhodopsins.
Related contents
- Lasers pick up good vibrations
- Using super-slow motion movie, scientists pin down the workings of a key proton pump
- Shining the spotlight on green fluorescent protein
Reference
- 1. Tahara, S., Kuramochi, H., Takeuchi, S. & Tahara, T. Protein dynamics preceding photoisomerization of the retinal chromophore in bacteriorhodopsin revealed by deep-UV femtosecond stimulated Raman spectroscopy. The Journal of Physical Chemistry Letters 10, 5422–5427 (2019). doi: 10.1021/acs.jpclett.9b02283
