Mar. 17, 2022 Research Highlight Biology
A viral inhibitor of cellular stress response shows therapeutic potential
A virus-produced protein that blocks the activation of a cellular-stress response could be used to save neurons in some neurodegenerative disorders
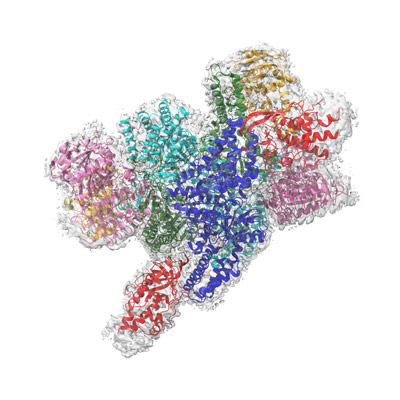 Figure 1: The structure of the complex of NSs with human eIF2B as determined by cryo-electron microscopy. © 2022 RIKEN Center for Biosystems Dynamic Research
Figure 1: The structure of the complex of NSs with human eIF2B as determined by cryo-electron microscopy. © 2022 RIKEN Center for Biosystems Dynamic Research
A viral protein that subdues the stress response of cells could offer an effective way to treat some neurological disorders, suggests a molecular-structural analysis by RIKEN biologists1.
When eukaryotic cells experience stressors such as infection by viruses, oxidative stress or insufficient nutrients, they activate the integrated stress response (ISR)—a signaling network in cells that helps cells to remain healthy under challenging circumstances. The ISR dramatically reconfigures the physiology of cells, largely shutting down protein production while activating genes that help cells respond appropriately to stressful stimuli.
However, several viruses have evolved strategies that sabotage this pathway. For example, the sandfly fever Sicilian virus produces a protein, NSs, that disrupts the ISR.
Takuhiro Ito of the RIKEN Center for Biosystems Dynamics Research, Shintaro Iwasaki of the RIKEN RNA Systems Biochemistry Laboratory, Yoshiho Ikeuchi of the University of Tokyo and their co-workers set out to determine the structure of NSs.
In healthy cells, a pair of cellular proteins known as eIF2 and eIF2B work together to facilitate the ribosome-mediated translation of RNA transcripts into proteins. During the ISR, however, eIF2 is enzymatically modified by the addition of a phosphate chemical group. This phosphorylated molecule exerts an inhibitory effect on eIF2B, which effectively grinds protein synthesis in cells to a halt.
The viral protein NSs is known to interact with eIF2B, and the researchers generated a high-resolution structure of this complex using a technique called cryo-electron microscopy (Fig. 1). Their analysis revealed that the viral protein protects eIF2B from the inhibitory effects exerted by phosphorylated eIF2, thereby allowing protein production to continue unabated.
Ito and his colleagues then confirmed that NSs can protect cultured rat and mouse neurons from damage or death resulting from chemically-induced stress. “NSs efficiently suppressed the ISR in these cells,” says Ito. “Indeed, the effects of this protein were even more potent than those of ISRIB, a previously developed drug that inhibits the ISR.”
These findings could have clinical value, as ISRIB has already demonstrated promise in preclinical testing as a treatment for conditions including traumatic brain injury, amyotrophic lateral sclerosis (ALS) and Down’s syndrome. ISR-induced cell death appears to be an important component of the pathology for these and other neurological conditions, and NSs could potentially outperform ISRIB in countering these toxic effects, Ito says.
“It will be very challenging to introduce NSs, which is a protein, into living neurons,” he explains. “However, if this difficulty can be overcome, I believe treatment with NSs will be very powerful for many neurodegenerative diseases.”
As a first step toward realizing actual clinical applications, the team has started experiments to examine whether NSs works effectively in mouse models of ALS.
Related contents
- How phosphorylation of eIF2 reduces protein synthesis
- Natural plant defense could lead to new personalized cancer therapy
Reference
- 1. Kashiwagi, K., Shichino, Y., Osaki, T., Sakamoto, A., Nishimoto, M., Takahashi, M., Mito, M., Weber, F., Ikeuchi, Y, Iwasaki, S. et al. eIF2B-capturing viral protein NSs suppresses the integrated stress response. Nature Communications 12, 7102 (2021). doi: 10.1038/s41467-021-27337-x
